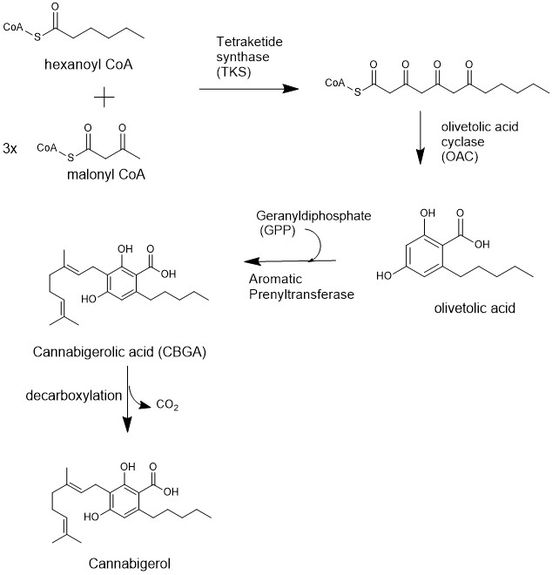
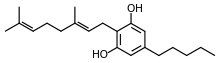
Cannabigerol
 |
|
 |
|
| Clinical data | |
|---|---|
| ATC code | none |
| Identifiers | |
|
|
| CAS Number | 25654-31-3 |
| PubChem (CID) | 5315659 |
| ChemSpider | 4474921 |
| ChEMBL | CHEMBL497318 |
| Chemical and physical data | |
| Formula | C21H32O2 |
| Molar mass | 316.48 g/mol |
| 3D model (Jmol) | Interactive image |
|
|
|
|
| |
|
Cannabigerol (CBG) is a cannabinoid found in the Cannabis genus of plants. Cannabigerol is found in higher concentrations in hemp rather than in varieties of Cannabis cultivated for high tetrahydrocannabinol (THC) content and their corresponding psychoactive properties. By the time most strains of cannabis are cultivated, they will have trace amounts of CBG, somewhere below 1%.[1]
Cannabigerol has been found to act as a high affinity α2-adrenergic receptor agonist, moderate affinity 5-HT1A receptor antagonist, and low affinity CB1 receptor antagonist.[2] It also binds to the CB2 receptor, but whether it acts as an agonist or antagonist at this site is unknown.[2]
Chemistry[edit]
It has two E/Z isomers.
Uses[edit]
Cannabigerol has been shown to relieve intraocular pressure, which may be of benefit in the treatment of glaucoma.[3][4] It can also be used to treat inflammatory bowel disease.[5] CBG can also inhibit the uptake of GABA in the brain, which can decrease anxiety and muscle tension.[6]
Legal status[edit]
CBG is not scheduled by Convention on Psychotropic Substances.
United States[edit]
CBG is not scheduled at the federal level in the United States.[7]
Biosynthesis[edit]
The biosynthesis of CBG begins loading a hexanoyl CoA onto a ketide synthetase assembly protein and subsequent condensation with three malonyl CoA molecules.[8] This polyketide is cyclized to olivetolic acid via an olivetolic cyclase, and then prenylated with a 10 carbon isoprenoid using Geranyldiphosphate (GPP) and an aromatic prenyltransferase protein to biosynthesize a precursor to CBG, cannabichomenic acid (CBCA).[9] CBCA is also a precursor to many cannabinoid compounds, including tetrahydrocannabinolic acid, or THCA. Finally, CBCA is cyclized into cannabigerolic acid (CBGA) using a cannabichromenic acid synthetase,[10] and decarboxylation yields cannabigerol.
See also[edit]
References[edit]
- ^ https://www.woahstork.com/blog/cbg-mother-of-cannabinoids/
- ^ a b Cascio MG, Gauson LA, Stevenson LA, Ross RA, Pertwee R (December 2009). "Evidence that the plant cannabinoid cannabigerol is a highly potent alpha(2)-adrenoceptor agonist and moderately potent 5HT receptor antagonist". British Journal of Pharmacology. 159 (1): 129–141. doi:10.1111/j.1476-5381.2009.00515.x. PMC 2823359
 . PMID 20002104.
. PMID 20002104. - ^ Colasanti, B. (1990). "A comparison of the ocular and central effects of delta 9-tetrahydrocannabinol and cannabigerol". Journal of ocular pharmacology. 6 (4): 259–269. doi:10.1089/jop.1990.6.259. PMID 1965836.
- ^ Colasanti, B.; Craig, C.; Allara, R. (1984). "Intraocular pressure, ocular toxicity and neurotoxicity after administration of cannabinol or cannabigerol". Experimental eye research. 39 (3): 251–259. doi:10.1016/0014-4835(84)90013-7. PMID 6499952.
- ^ "Beneficial effect of the non-psychotropic plant cannabinoid cannabigerol on experimental inflammatory bowel disease.". Biochem Pharmacol. 85 (9): 1306–16. May 2013. doi:10.1016/j.bcp.2013.01.017. PMID 23415610.
- ^ Medical Jane, Cannabigerol (CBG): A Minor Cannabinoid With A Major Impact
- ^ §1308.11 Schedule I.
- ^ Page Et. Al, Jonathan (2012). "Identification of olivetolic acid cyclase from Cannabis sativa reveals a unique catalytic route to plant polyketides". PNAS. 109: 12811–6. doi:10.1073/pnas.1200330109. PMID 22802619.
- ^ Meinhart H,, Zenk Et. Al (1998). "Prenylation of olivetolate by a hemp transferase yields cannabigerolic acid, the precursor of tetrahydrocannabinol". FEBS Letters. 427: 283–285. doi:10.1016/s0014-5793(98)00450-5.
- ^ Shoyama, Yukihiro (1997). "Enzymological Evidence for Cannabichromenic Acid Biosynthesis". Journal of Natural Products. 60: 854–857. doi:10.1021/np970210y.
| This drug article relating to the nervous system is a stub. You can help Wikipedia by expanding it. |