Falcarinol
 |
|
| Names | |
|---|---|
| IUPAC name
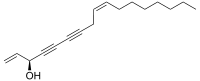
(3S,9Z)-Heptadeca-1,9-diene-4,6-diyn-3-ol
|
|
| Other names
Carotatoxin
|
|
| Identifiers | |
| 21852-80-2 |
|
| 3D model (Jmol) | Interactive image |
| ChemSpider | 4580244 |
| KEGG | C08450 |
| PubChem | 5469789 |
|
|
|
|
| Properties | |
| C17H24O | |
| Molar mass | 244.38 g·mol−1 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |
Falcarinol (carotatoxin) is a natural pesticide and fatty alcohol found in carrots (Daucus carota), red ginseng (Panax ginseng) and ivy. In carrots, it occurs in a concentration of approximately 2 mg/kg.[1][2] As a toxin, it protects roots from fungal diseases, such as liquorice rot that causes black spots on the roots during storage.
Chemistry[edit]
Falcarinol is a polyyne with two carbon-carbon triple bonds and two double bonds.[3] Falcarinol can cause allergic and irritant contact dermatitis.[4] It is structurally related to the oenanthotoxin and cicutoxin.
Biological effects[edit]
It was shown that falcarinol acts as a covalent cannabinoid receptor type 1 inverse agonist and blocks the effect of anandamide in keratinocytes, leading to pro-allergic effects in human skin.[5]
Preliminary research in animal models suggest that falcarinol may have a protective effect against certain types of cancer. Laboratory rats fed a diet containing raw carrots or isolated falcarinol were a third less likely to develop full-scale tumors induced by azoxymethane than those in a control group.[6]
Normal consumption of carrots doesn't cause any toxic effect in humans. However, when falcarinol is delivered in high doses to laboratory animals, it causes neurotoxical problems.[7]
See also[edit]
References[edit]
- ^ Crosbya, D. G.; Aharonson, N. (1967). "The Structure of Carotatoxin, a Natural Toxicant From Carrot". Tetrahedron. 23 (1): 465–472. doi:10.1016/S0040-4020(01)83330-5.
- ^ Badui (1988). Diccionario de Tecnología de Alimentos. D. F. Mexico: Alhambra Mexicana. ISBN 968-444-071-5.
- ^ S. G. Yates; R. E. England (1982). "Isolation and analysis of carrot constituents: myristicin, falcarinol, and falcarindiol". Journal of Agricultural and Food Chemistry. 30 (2): 317–320. doi:10.1021/jf00110a025.
- ^ S. Machado; E. Silva; A. Massa (2002). "Occupational allergic contact dermatitis from falcarinol". Contact Dermatitis. 47 (2): 109–125. doi:10.1034/j.1600-0536.2002.470210_5.x.
- ^ M. Leonti; S. Raduner; L. Casu; F. Cottiglia; C. Floris; KH. Altmann; J. Gertsch (2010). "Falcarinol is a covalent cannabinoid CB1 receptor antagonist and induces pro-allergic effects in skin". Biochemical Pharmacology. 79 (12): 1815–1826. doi:10.1016/j.bcp.2010.02.015. PMID 20206138.
- ^ M. Kobæk-Larsen; L. P. Christensen; W. Vach; J. Ritskes-Hoitinga; K. Brandt (2005). "Inhibitory Effects of Feeding with Carrots or (−)-Falcarinol on Development of Azoxymethane-Induced Preneoplastic Lesions in the Rat Colon". Journal of Acricultural and Food Chemistry. 53 (5): 1823–1827. doi:10.1021/jf048519s. PMID 15740080.
- ^ Deshpande (2002). Handbook of Food Toxicology. Hyderabad, India: CRC Press. ISBN 978-0-8247-0760-6.
External links[edit]
- Carrots may help ward off cancer, BBC News
- Cancer boost from whole carrots, BBC News