Indiplon
 |
|
| Systematic (IUPAC) name | |
|---|---|
|
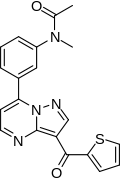
N-methyl-N-[3-[3-(thiophene-2-carbonyl)
pyrazolo[5,1-b]pyrimidin-7-yl]phenyl]acetamide |
|
| Clinical data | |
| Routes of administration |
Oral |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | 325715-02-4 |
| ATC code | none |
| PubChem | CID 6450813 |
| IUPHAR/BPS | 4221 |
| ChemSpider | 4953363 |
| UNII | 8BT63DA42E |
| KEGG | D02640 |
| ChEMBL | CHEMBL262075 |
| Chemical data | |
| Formula | C20H16N4O2S |
| Molar mass | 376.0993 g/mol |
| 3D model (Jmol) | Interactive image |
|
|
|
|
| |
|
Indiplon (INN and USAN) is a nonbenzodiazepine, hypnotic sedative that was developed in 2 formulations - an immediate release product for sleep onset and a modified-release (a.k.a. controlled-release or extended-release) version for sleep maintenance.
Mode of action[edit]
Indiplon works by enhancing the action of the inhibitory neurotransmitter GABA, like most other nonbenzodiazepine sedatives. It primarily binds to the α1 subunits of the GABAA receptors in the brain.[1]
History[edit]
Indiplon was discovered at Lederle Laboratories (which was later acquired by Wyeth) in the 1980s and was called CL 285,489.[2]:454 In 1998 Lederle licensed it, along with other early stage drug candidates, to DOV Pharmaceutical, a startup formed by former Lederle employees, and Dov exclusively sublicensed its rights in the drug to Neurocrine Biosciences in that same year.[2] In 2002, Neurocrine entered into an agreement with Pfizer to develop the drug.[2]
Indiplon was originally scheduled for release in 2007, when Sanofi-Aventis' popular hypnotic zolpidem lost its patent rights in the United States and thus became available as a much less expensive generic. In 2002, Neurocrine Biosciences had entered into an agreement with Pfizer to co-market indiplon in the US, in a deal worth a potential $400mn.[3] However, following the issuing of a non-approvable letter for the modified-release 15 mg formulation and an approvable letter with stipulations for the 5 mg and 10 mg immediate-release version by the FDA in May 2006,[4] Pfizer ended its relationship with Neurocrine.[5] Neurocrine's stock price dropped 60% on the news.[6]
Following a resubmission, the FDA in December 2007 deemed Neurocrine's new drug application (NDA) 'approvable' in the 5 and 10 mg formulations,[7] but requested new studies as a prerequisite to approval, including a clinical trial in the elderly, a safety study comparing adverse effects to those of similarly marketed drugs, and a preclinical study examining indiplon's safety in the third trimester of pregnancy.[8]
Following the 2007 FDA letter, Neurocrine decided to discontinue all clinical and marketing development of Indiplon in the United States.[7][8]
References[edit]
- ^ Petroski RE, Pomeroy JE, Das R, et al. (April 2006). "Indiplon is a high-affinity positive allosteric modulator with selectivity for alpha1 subunit-containing GABAA receptors" (PDF). J. Pharmacol. Exp. Ther. 317 (1): 369–77. doi:10.1124/jpet.105.096701. PMID 16399882.
- ^ a b c Neubauer, DN. "Indiplon". pp 453-464 in GABA and Sleep: Molecular, Functional and Clinical Aspects. Eds Jaime M. Monti, Seithikurippu Ratnas Pandi-Perumal, Hanns Möhler. Springer Science & Business Media, 2010 ISBN 9783034602266
- ^ San Diego's Neurocrine Biosciences Scores Second Big Deal in Two Days - The Motley Fool, 18 June 2010
- ^ Neurocrine's FDA Nightmare - TheStreet.com, 16 May 2006
- ^ Pfizer Drops Neurocrine Deal - TheStreet.com, 22 June 2006
- ^ Neurocrine stock price plunges 60 percent:FDA's mixed review of sleeping pill Indiplon could threaten Pfizer-Neurocrine partnership - CNN Money, 15 May 2006
- ^ a b "Neurocrine Receives Approvable Letter for Indiplon Capsules with Additional Safety and Efficacy Data Required by FDA" (Press release). Neurocrine Biosciences, Inc. 2007-12-13. Retrieved 2007-12-13.
- ^ a b "Additional Pipeline Projects". Neurocrine. 2012-02-16. Archived from the original on 2012-02-20. Retrieved 2014-06-24.
External links[edit]
- Neurocrine's drug pipeline page referencing indiplon
- 2004 press release announcing Neurocrine's new product, Indiplon
- Pfizer
- GenomeNet Entry: D02640