- published: 07 Oct 2013
- views: 380626
-
remove the playlistAmorphous Solid
-
remove the playlistLatest Videos
-
remove the playlistLongest Videos
- remove the playlistAmorphous Solid
- remove the playlistLatest Videos
- remove the playlistLongest Videos
- published: 27 Apr 2010
- views: 45322
- published: 04 Dec 2012
- views: 15166
- published: 07 Jan 2014
- views: 5847
- published: 04 Jul 2015
- views: 101986
- published: 07 Sep 2010
- views: 5156
- published: 08 Dec 2014
- views: 1738
- published: 19 Feb 2014
- views: 1872
Amorphous solid
In condensed matter physics and materials science, an amorphous (from the Greek a, without, morphé, shape, form) or non-crystalline solid is a solid that lacks the long-range order characteristic of a crystal. In some older books, the term has been used synonymously with glass. Nowadays, "amorphous solid" is considered to be the overarching concept, and glass the more special case: A glass is an amorphous solid that exhibits a glass transition. Polymers are often amorphous. Other types of amorphous solids include gels, thin films, and nanostructured materials such as glass.
Amorphous materials have an internal structure made of interconnected structural blocks. Whether a material is liquid or solid depends primarily on the connectivity between its elementary building blocks so that solids are characterized by a high degree of connectivity whereas structural blocks in fluids have lower connectivity (see figure on amorphous material states).
Nano-structured materials
Even amorphous materials have some shortrange order at the atomic length scale due to the nature of chemical bonding (see structure of liquids and glasses for more information on non-crystalline material structure). Furthermore, in very small crystals a large fraction of the atoms are the crystal; relaxation of the surface and interfacial effects distort the atomic positions, decreasing the structural order. Even the most advanced structural characterization techniques, such as x-ray diffraction and transmission electron microscopy, have difficulty in distinguishing between amorphous and crystalline structures on these length scales.
This article is licensed under the Creative Commons Attribution-ShareAlike 3.0 Unported License, which means that you can copy and modify it as long as the entire work (including additions) remains under this license.
- Loading...
-
 9:18
9:18Doing Solids: Crash Course Chemistry #33
Doing Solids: Crash Course Chemistry #33Doing Solids: Crash Course Chemistry #33
You can directly support Crash Course at http://www.subbable.com/crashcourse Subscribe for as little as $0 to keep up with everything we're doing. Also, if you can afford to pay a little every month, it really helps us to continue producing great content. In which Hank blows our minds with the different kinds of Solids out there and talks about why they're all different and have different properties. Today, you'll learn about amorphous and crystalline solids, types of crystalline solids, types of crystalline atomic solids, properties of each type of solid, and that the properties depend on the bond types. ***** AND NOW, A SUBBABLE MESSAGE! ***** "Don't forget to be awesome, Dad! (For Tony) (Benji assisted)" - Daniel Smith -- Table of Contents Amorphous and Crystalline Solids 1:27 Types of Crystalline Solids 4:07 Types of Crystalline Atomic Solids 5:17 Properties of Each Type of Solid 4:16 Properties Depend on Bond Types 6:17 -- Want to find Crash Course elsewhere on the internet? Facebook - http://www.facebook.com/YouTubeCrashCourse Twitter - http://www.twitter.com/TheCrashCourse Tumblr - http://thecrashcourse.tumblr.com Support CrashCourse on Subbable: http://subbable.com/crashcourse -
 2:03
2:03Crystalline And Amorphous Solids
Crystalline And Amorphous SolidsCrystalline And Amorphous Solids
Follow us at: https://plus.google.com/+tutorvista/ Check us out at http://www.tutorvista.com/content/physics/physics-iii/solids-and-fluids/amorphous-solids.php Crystalline And Amorphous Solids Amorphous solids and crystalline solids if the size of the crystals is very small. Even amorphous materials have some short-range order at the atomic length scale due the nature of chemical bonding. Furthermore, in very small crystals a large fraction of the atoms are located at or near the surface of the crystal; relaxation of the surface and interfacial effects distort the atomic positions, decreasing the structural order. Even the most advanced structural characterization techniques, such as x-ray diffraction and transmission electron microscopy, have difficulty in distinguishing between amorphous and crystalline structures on these length scales.The transition from the liquid state to the glass, at a temperature below the equilibrium melting point of the material, is called the glass transition. The glass transition temperature, Tg, is the temperature at which an amorphous solid, such as glass or a polymer, becomes brittle on cooling, or soft on heating. More specifically, it defines a pseudo second order phase transition in which a supercooled melt yields, on cooling, a glassy structure and properties similar to those of crystalline materials e.g. of an isotropic solid material. Tg is usually applicable to wholly or partially amorphous solids such as common glasses and plastics (organic polymers). Below the glass transition temperature, Tg, amorphous solids are in a glassy state and most of their joining bonds are intact. In inorganic glasses, with increased temperature more and more joining bonds are broken by thermal fluctuations so that broken bonds (termed configurons) begin to form clusters. Above Tg these clusters become macroscopic large facilitating the flow of material. In organic polymers, secondary, non-covalent bonds between the polymer chains become weak above Tg. Above Tg glasses and organic polymers become soft and capable of plastic deformation without fracture. This behavior is one of the things which make most plastics useful . It is important to note that the glass transition temperature is a kinetic parameter, and thus parametrically depends on the melt cooling rate. Thus the slower the melt cooling rate, the lower Tg. In addition, Tg depends on the measurement conditions, which are not universally defined . The glass transition temperature is approximately the temperature at which the viscosity of the liquid exceeds a certain value (about 1012 Pa•s). The transition temperature depends on cooling rate, with the glass transition occurring at higher temperatures for faster cooling rates. The precise nature of the glass transition is the subject of ongoing research. While it is clear that the glass transition is not a first-order thermodynamic transition (such as melting), there is debate as to whether it is a higher-order transition such percolation type transformation , or merely a kinetic effect. The process of forming a crystalline structure from a fluid or from materials dissolved in the fluid is often referred to as crystallization. In the old example referenced by the root meaning of the word crystal, water being cooled undergoes a phase change from liquid to solid beginning with small ice crystals that grow until they fuse, forming a polycrystalline structure. The physical properties of the ice depend on the size and arrangement of the individual crystals, or grains, and the same may be said of metals solidifying from a molten state.Which crystal structure the fluid will form depends on the chemistry of the fluid, the conditions under which it is being solidified, and also on the ambient pressure. While the cooling process usually results in the generation of a crystalline material, under certain conditions, the fluid may be frozen in a noncrystalline state. In most cases, this involves cooling the fluid so rapidly that atoms cannot travel to their lattice sites before they lose mobility. A noncrystalline material, which has no long-range order, is called an amorphous, vitreous, or glassy material. Please like our facebook page http://www.facebook.com/tutorvista -
 5:10
5:10Crystalline Solids and Amorphous Solids
Crystalline Solids and Amorphous SolidsCrystalline Solids and Amorphous Solids
Donate here: http://www.aklectures.com/donate.php Website video link: http://www.aklectures.com/lecture/crystalline-solids-and-amorphous-solids Facebook link: https://www.facebook.com/aklectures Website link: http://www.aklectures.com -
 2:35
2:35Amorphous Solids
Amorphous SolidsAmorphous Solids
Learn about amorphous solids and their unique properties. Also get a better understanding of their rigid form. Make sure you are ready for test day. Visit: http://www.mometrix.com/academy/amorphous-solids/ Subscribe to more free test preparation videos: http://bit.ly/1dJH1yb Follow Mometrix Academy on Pinterest: http://bit.ly/1hZE2Jj Review our free test prep directory: http://bit.ly/1hZE2Jj Learn more About Us: http://bit.ly/1ewIADC #MometrixAcademy #AmorphousSolids -
 3:22
3:22Is Glass Really A Solid?
Is Glass Really A Solid?Is Glass Really A Solid?
Of course glass is a solid… right? Could it possibly be a liquid? Read More: Physicists shatter stubborn mystery of how glass forms http://www.eurekalert.org/pub_releases/2015-06/uow-pss062915.php “A physicist at the University of Waterloo is among a team of scientists who have described how glasses form at the molecular level and provided a possible solution to a problem that has stumped scientists for decades.” Fact or Fiction?: Glass Is a (Supercooled) Liquid http://www.scientificamerican.com/article/fact-fiction-glass-liquid/ “Glass, however, is actually neither a liquid—supercooled or otherwise—nor a solid. It is an amorphous solid—a state somewhere between those two states of matter. “ What makes glass transparent? http://science.howstuffworks.com/question4041.htm ____________________ DNews is dedicated to satisfying your curiosity and to bringing you mind-bending stories & perspectives you won't find anywhere else! New videos twice daily. Watch More DNews on TestTube http://testtube.com/dnews Subscribe now! http://www.youtube.com/subscription_center?add_user=dnewschannel DNews on Twitter http://twitter.com/dnews Trace Dominguez on Twitter https://twitter.com/tracedominguez Julia Wilde on Twitter https://twitter.com/julia_sci DNews on Facebook https://facebook.com/DiscoveryNews DNews on Google+ http://gplus.to/dnews Discovery News http://discoverynews.com Download the TestTube App: http://testu.be/1ndmmMq -
 8:52
8:52Amorphous Solid lab.wmv
Amorphous Solid lab.wmvAmorphous Solid lab.wmv
This lab shows the procedure for making the amorphous solid : "Gluep" -
 2:37
2:37Understand the differences between Crystalline and Amorphous Solids
Understand the differences between Crystalline and Amorphous SolidsUnderstand the differences between Crystalline and Amorphous Solids
There are two main classes of solids namely crystalline and amorphous. Click on the links to know more. Crystalline solids have a regular and repetitive patterns of atoms, molecules or ions. They are symmetrical shape flat smooth and plane faces which meet at a characteristic angle. They are classified as single crystal and poly crystalline crystals. If the periodicity of pattern is extended throughout the pattern then it is called single crystal solid. In poly crystalline crystals the periodicity of the pattern is interrupted at the boundaries. Crystalline solids have sharp melting points and all bonds have same strength. Diamond, rock salt, mica and sugar belong to this category. In amorphous solids, like liquids, the atoms and molecules are not arranged in an orderly manner. But in amorphous solids atoms are rigidly fixed and they are allowed to move as in liquids. Glass is an amorphous solid. -
 1:58
1:58Amorphous Solid Lab Day - 2.mp4
Amorphous Solid Lab Day - 2.mp4Amorphous Solid Lab Day - 2.mp4
Testing the Properties of an Amorphous Solid -
 67:20
67:20Amorphous Solid Dispersion Formulations Using The Spray Dry Process
Amorphous Solid Dispersion Formulations Using The Spray Dry ProcessAmorphous Solid Dispersion Formulations Using The Spray Dry Process
Amorphous solid dispersion technology has been developed to be a preferred formulation option to improve solubility and dissolution rate of insoluble compounds with a wide range of applicability. When amorphous compounds are delivered orally, their solubility advantage can translate into significantly enhanced bioavailability. This webinar will discuss the application of amorphous solid dispersions via the spray drying process in preclinical formulation identification, including stability and performance considerations. Illustrative case studies will be presented. -
 58:36
58:36Hot-Melt Extrusion Fundamentals: Processing of Amorphous Solid Dispersions for Poorly Soluble Drugs
Hot-Melt Extrusion Fundamentals: Processing of Amorphous Solid Dispersions for Poorly Soluble DrugsHot-Melt Extrusion Fundamentals: Processing of Amorphous Solid Dispersions for Poorly Soluble Drugs
Bend Research is the leader in drug delivery technologies and formulation development. We're known for enhancing the bioavailability of low-solubility compounds and modified release dosage form development from discovery to commercialization.
-

Doing Solids: Crash Course Chemistry #33
You can directly support Crash Course at http://www.subbable.com/crashcourse Subscribe for as little as $0 to keep up with everything we're doing. Also, if you can afford to pay a little every month, it really helps us to continue producing great content. In which Hank blows our minds with the different kinds of Solids out there and talks about why they're all different and have different properties. Today, you'll learn about amorphous and crystalline solids, types of crystalline solids, types of crystalline atomic solids, properties of each type of solid, and that the properties depend on the bond types. ***** AND NOW, A SUBBABLE MESSAGE! ***** "Don't forget to be awesome, Dad! (For Tony) (Benji assisted)" - Daniel Smith -- Table of Contents Amorphous and Crystalline Solids 1:27 Typ... -

Crystalline And Amorphous Solids
Follow us at: https://plus.google.com/+tutorvista/ Check us out at http://www.tutorvista.com/content/physics/physics-iii/solids-and-fluids/amorphous-solids.php Crystalline And Amorphous Solids Amorphous solids and crystalline solids if the size of the crystals is very small. Even amorphous materials have some short-range order at the atomic length scale due the nature of chemical bonding. Furthermore, in very small crystals a large fraction of the atoms are located at or near the surface of the crystal; relaxation of the surface and interfacial effects distort the atomic positions, decreasing the structural order. Even the most advanced structural characterization techniques, such as x-ray diffraction and transmission electron microscopy, have difficulty in distinguishing between amorp... -

Crystalline Solids and Amorphous Solids
Donate here: http://www.aklectures.com/donate.php Website video link: http://www.aklectures.com/lecture/crystalline-solids-and-amorphous-solids Facebook link: https://www.facebook.com/aklectures Website link: http://www.aklectures.com -

Amorphous Solids
Learn about amorphous solids and their unique properties. Also get a better understanding of their rigid form. Make sure you are ready for test day. Visit: http://www.mometrix.com/academy/amorphous-solids/ Subscribe to more free test preparation videos: http://bit.ly/1dJH1yb Follow Mometrix Academy on Pinterest: http://bit.ly/1hZE2Jj Review our free test prep directory: http://bit.ly/1hZE2Jj Learn more About Us: http://bit.ly/1ewIADC #MometrixAcademy #AmorphousSolids -

Is Glass Really A Solid?
Of course glass is a solid… right? Could it possibly be a liquid? Read More: Physicists shatter stubborn mystery of how glass forms http://www.eurekalert.org/pub_releases/2015-06/uow-pss062915.php “A physicist at the University of Waterloo is among a team of scientists who have described how glasses form at the molecular level and provided a possible solution to a problem that has stumped scientists for decades.” Fact or Fiction?: Glass Is a (Supercooled) Liquid http://www.scientificamerican.com/article/fact-fiction-glass-liquid/ “Glass, however, is actually neither a liquid—supercooled or otherwise—nor a solid. It is an amorphous solid—a state somewhere between those two states of matter. “ What makes glass transparent? http://science.howstuffworks.com/question4041.htm ___... -

Amorphous Solid lab.wmv
This lab shows the procedure for making the amorphous solid : "Gluep" -

Understand the differences between Crystalline and Amorphous Solids
There are two main classes of solids namely crystalline and amorphous. Click on the links to know more. Crystalline solids have a regular and repetitive patterns of atoms, molecules or ions. They are symmetrical shape flat smooth and plane faces which meet at a characteristic angle. They are classified as single crystal and poly crystalline crystals. If the periodicity of pattern is extended throughout the pattern then it is called single crystal solid. In poly crystalline crystals the periodicity of the pattern is interrupted at the boundaries. Crystalline solids have sharp melting points and all bonds have same strength. Diamond, rock salt, mica and sugar belong to this category. In amorphous solids, like liquids, the atoms and molecules are not arranged in an orderly manner. But in amor... -

Amorphous Solid Lab Day - 2.mp4
Testing the Properties of an Amorphous Solid -

Amorphous Solid Dispersion Formulations Using The Spray Dry Process
Amorphous solid dispersion technology has been developed to be a preferred formulation option to improve solubility and dissolution rate of insoluble compounds with a wide range of applicability. When amorphous compounds are delivered orally, their solubility advantage can translate into significantly enhanced bioavailability. This webinar will discuss the application of amorphous solid dispersions via the spray drying process in preclinical formulation identification, including stability and performance considerations. Illustrative case studies will be presented. -

Hot-Melt Extrusion Fundamentals: Processing of Amorphous Solid Dispersions for Poorly Soluble Drugs
Bend Research is the leader in drug delivery technologies and formulation development. We're known for enhancing the bioavailability of low-solubility compounds and modified release dosage form development from discovery to commercialization.
Doing Solids: Crash Course Chemistry #33
- Order: Reorder
- Duration: 9:18
- Updated: 07 Oct 2013
- views: 380626
- published: 07 Oct 2013
- views: 380626
Crystalline And Amorphous Solids
- Order: Reorder
- Duration: 2:03
- Updated: 27 Apr 2010
- views: 45322
- published: 27 Apr 2010
- views: 45322
Crystalline Solids and Amorphous Solids
- Order: Reorder
- Duration: 5:10
- Updated: 04 Dec 2012
- views: 15166
- published: 04 Dec 2012
- views: 15166
Amorphous Solids
- Order: Reorder
- Duration: 2:35
- Updated: 07 Jan 2014
- views: 5847
- published: 07 Jan 2014
- views: 5847
Is Glass Really A Solid?
- Order: Reorder
- Duration: 3:22
- Updated: 04 Jul 2015
- views: 101986
- published: 04 Jul 2015
- views: 101986
Amorphous Solid lab.wmv
- Order: Reorder
- Duration: 8:52
- Updated: 07 Sep 2010
- views: 5156
- published: 07 Sep 2010
- views: 5156
Understand the differences between Crystalline and Amorphous Solids
- Order: Reorder
- Duration: 2:37
- Updated: 08 Dec 2014
- views: 1738
- published: 08 Dec 2014
- views: 1738
Amorphous Solid Lab Day - 2.mp4
- Order: Reorder
- Duration: 1:58
- Updated: 08 Sep 2010
- views: 2015
Amorphous Solid Dispersion Formulations Using The Spray Dry Process
- Order: Reorder
- Duration: 67:20
- Updated: 19 Feb 2014
- views: 1872
- published: 19 Feb 2014
- views: 1872
Hot-Melt Extrusion Fundamentals: Processing of Amorphous Solid Dispersions for Poorly Soluble Drugs
- Order: Reorder
- Duration: 58:36
- Updated: 04 Nov 2015
- views: 318
-

Hydrogenated Amorphous Silicon Cambridge Solid State Science Series
-

Difference Between Amorphous and Crystalline Solid
Watch More Difference Between Related Videos: Difference Between Nerve and Neuron https://www.youtube.com/watch?v=cQy2lyydP2M Difference Between Emission and Radiation https://www.youtube.com/watch?v=QJJ6NMf45uA Difference Between Lytic and Lysogenic https://www.youtube.com/watch?v=1nPSSiubx4g Difference Between Calories and Carbs https://www.youtube.com/watch?v=KDVNL7BB0qk Difference Between Alpha Beta and Gamma Radiation https://www.youtube.com/watch?v=pQMlVb7Tf6M Difference Between Transvestites and Transsexuals https://www.youtube.com/watch?v=wvY6Sf06890 Difference Between Dicot and Monocot https://www.youtube.com/watch?v=gl5ejOCLjvA Difference Between Mole and Molarity https://www.youtube.com/watch?v=IIRXKujRJdQ Difference Between Reaction and Reflex https://www.youtube.com/watch?v=_a... -

9th (Punjab Board) Chemistry Chapter 5.4 - LEC 3 Amorphous Solids
Share With Class Mates & DON'T Forget To Click Subscribe Button Thank You !!! For More Videos Click Here: http://adf.ly/1XUqOG All Subjects Available on DVD Order All Over Pakistan http://adf.ly/1YUys5 Facebook https://www.facebook.com/maktab.production/ Twitter https://twitter.com/MaktabTeacher -

Define Solid | Types Of Waste | Amorphous Solid | Network Solid | Molecular Solids
Solids have a defined shape and volume more over solids are classified as ionic solid ,mettalic solid ,network atomic solid ,molecular solid and amorphous solids .Ionic solids form when electrostatic attraction sticks together anions and cations to form a crystal lattice.mettalic solids are Positively charged nuclei of metal atoms are held together by valence electrons to form metallic solids. Network atomic solid also is known simply as a network solid.Atomic solids form when weak London dispersion forces bind atoms of cold noble gases.molecular solid Covalent molecules are held together by intermolecular forces to form molecular solids.Amorphous solids may be soft and rubbery . -

Solid State NCERT
Solid State Chemistry NCERT text book explained including crystalline and amorphous solids, their characteristics like anisotropy and cleave property. http://chemistryconcept.com -

Amorphous solid
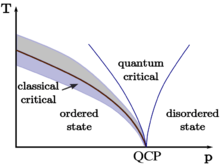
Amorphous solid In condensed matter physics and materials science, an amorphous (from the Greek a, without, morphé, shape, form) or non-crystalline solid is a solid that lacks the long-range order characteristic of a crystal.In some older books, the term has been used synonymously with glass. =======Image-Copyright-Info======== License: Creative Commons Attribution-Share Alike 3.0 (CC BY-SA 3.0) LicenseLink: http://creativecommons.org/licenses/by-sa/3.0 Author-Info: DG85 Image Source: https://en.wikipedia.org/wiki/File:QuantumPhaseTransition.png =======Image-Copyright-Info======== -Video is targeted to blind users Attribution: Article text available under CC-BY-SA image source in video https://www.youtube.com/watch?v=XcxTm8ILygs -

Amorphous solid - Video Learning - WizScience.com
In condensed matter physics and materials science, an "amorphous" or "non-crystalline solid" is a solid that lacks the long-range order characteristic of a crystal. In some older books, the term has been used synonymously with glass. Nowadays, "amorphous solid" is considered to be the overarching concept, and glass the more special case: A glass is an amorphous solid that exhibits a glass transition. Polymers are often amorphous. Other types of amorphous solids include gels, thin films, and nanostructured materials such as glass. Amorphous materials have an internal structure made of interconnected structural blocks. Whether a material is liquid or solid depends primarily on the connectivity between its elementary building blocks so that solids are characterized by a high degree of conn... -

Difference between Crystalline and Amorphous Solids
Class 12: Chemistry: Solid State-I: Difference between Crystalline and Amorphous Solids
Hydrogenated Amorphous Silicon Cambridge Solid State Science Series
- Order: Reorder
- Duration: 0:19
- Updated: 30 Jun 2016
- views: 0
- published: 30 Jun 2016
- views: 0
Difference Between Amorphous and Crystalline Solid
- Order: Reorder
- Duration: 0:47
- Updated: 07 May 2016
- views: 52
- published: 07 May 2016
- views: 52
9th (Punjab Board) Chemistry Chapter 5.4 - LEC 3 Amorphous Solids
- Order: Reorder
- Duration: 8:10
- Updated: 04 May 2016
- views: 9
- published: 04 May 2016
- views: 9
Define Solid | Types Of Waste | Amorphous Solid | Network Solid | Molecular Solids
- Order: Reorder
- Duration: 9:38
- Updated: 19 Apr 2016
- views: 8
- published: 19 Apr 2016
- views: 8
Solid State NCERT
- Order: Reorder
- Duration: 15:59
- Updated: 12 Mar 2016
- views: 60
- published: 12 Mar 2016
- views: 60
Amorphous solid
- Order: Reorder
- Duration: 4:49
- Updated: 22 Jan 2016
- views: 9
- published: 22 Jan 2016
- views: 9
Amorphous solid - Video Learning - WizScience.com
- Order: Reorder
- Duration: 2:31
- Updated: 10 Sep 2015
- views: 81
- published: 10 Sep 2015
- views: 81
Difference between Crystalline and Amorphous Solids
- Order: Reorder
- Duration: 0:05
- Updated: 24 Jun 2015
- views: 110
- published: 24 Jun 2015
- views: 110
-

How to : Crystalline and Amorphous Solid # Lecture # ClassXII
Crystalline and Amorphous Solid - Solid State - GPC 20.2 For More Online Videos Click Here : http://www.youtube.com/user/GuruprakashAcademy For Latest Updates Subscribe to our channel : http://www.youtube.com/subscription_center?add_user=GuruprakashAcademy If you have any questions or suggestions, leave them in the comments Please hit "LIKE" if it's a GOOD Lecture :-) -

Hot-Melt Extrusion of Amorphous Solid Dispersions for Bioavailability Enhancement
A large majority of active pharmaceutical ingredients (API) currently in development have limited bioavailability due to low solubility and dissolution rates (BCS/DCS IIa and IIb). Bend Research, part of Capsugel Dosage Form Soultions, has a multitude of established technologies and rigorous selection criteria to address this problem on a per API basis. One approach, hot-melt extrusion (HME) of amorphous solid dispersions is a mature and commercial ready technology platform. The company uses an integrated process and formulation approach to HME that balances the target absorption profile, manufacturability, physical and chemical stability, and API availability to develop a progressable solid dispersion drug intermediate and dosage form that is robust and scalable. Strategies and case stu... -

13.1 Amorphous vs crystalline solids & classification of solids
-

-

Role of Excipients in Design of Solid Amorphous Dispersions - Thomas Durig
For more information, please visit us at: http://www.ashland.com/pharmaceutical/learning-center -

Bravais Lattice ,Amorphous and Crystalline Solid in Solid State for IIT
Solid State,Amorphous and Crystalline Solids,Crystal terminology,Bravais Lattice and IIT question -

Plasticity and Material Failure in Amorphous Solids(Chandrasekhar Lecture II) by Itamar Procaccia
Discussion Meeting: Nonlinear Physics of Disordered Systems: From Amorphous Solids to Complex Flows URL: http://www.icts.res.in/discussion_meeting/NPDS2015/ Dates: Monday 06 Apr, 2015 - Wednesday 08 Apr, 2015 Description: In recent years significant progress has been made in the physics of disordered systems. As is usual in nonlinear physics one can rarely employ standard methods - nonlinear systems require specialized thinking to provide useful progress. Nevertheless some generic techniques like scaling on the one hand and bifurcation theory on the other can find powerful ramifications in the explored issues. In this discussion meeting we will focus on recent advances in this field and the outstanding open questions. -

Lecture 20 Amorphous Solids, Glass Formation, Inorganic Glasses Silicates
How to : Crystalline and Amorphous Solid # Lecture # ClassXII
- Order: Reorder
- Duration: 20:10
- Updated: 27 Oct 2012
- views: 4526
- published: 27 Oct 2012
- views: 4526
Hot-Melt Extrusion of Amorphous Solid Dispersions for Bioavailability Enhancement
- Order: Reorder
- Duration: 57:15
- Updated: 24 Apr 2014
- views: 5234
- published: 24 Apr 2014
- views: 5234
13.1 Amorphous vs crystalline solids & classification of solids
- Order: Reorder
- Duration: 43:03
- Updated: 04 Dec 2013
- views: 3608
- published: 04 Dec 2013
- views: 3608
Lec 20 | MIT 3.091 Introduction to Solid State Chemistry
- Order: Reorder
- Duration: 50:14
- Updated: 29 Apr 2008
- views: 44046
Role of Excipients in Design of Solid Amorphous Dispersions - Thomas Durig
- Order: Reorder
- Duration: 26:50
- Updated: 07 Nov 2014
- views: 1337
- published: 07 Nov 2014
- views: 1337
Bravais Lattice ,Amorphous and Crystalline Solid in Solid State for IIT
- Order: Reorder
- Duration: 37:47
- Updated: 26 Jan 2013
- views: 5605
- published: 26 Jan 2013
- views: 5605
Plasticity and Material Failure in Amorphous Solids(Chandrasekhar Lecture II) by Itamar Procaccia
- Order: Reorder
- Duration: 75:47
- Updated: 11 May 2015
- views: 338
- published: 11 May 2015
- views: 338
Lecture 20 Amorphous Solids, Glass Formation, Inorganic Glasses Silicates
- Order: Reorder
- Duration: 50:14
- Updated: 25 May 2014
- views: 380
- published: 25 May 2014
- views: 380
- Playlist
- Chat
- Playlist
- Chat

Doing Solids: Crash Course Chemistry #33
- Report rights infringement
- published: 07 Oct 2013
- views: 380626

Crystalline And Amorphous Solids
- Report rights infringement
- published: 27 Apr 2010
- views: 45322

Crystalline Solids and Amorphous Solids
- Report rights infringement
- published: 04 Dec 2012
- views: 15166

Amorphous Solids
- Report rights infringement
- published: 07 Jan 2014
- views: 5847

Is Glass Really A Solid?
- Report rights infringement
- published: 04 Jul 2015
- views: 101986

Amorphous Solid lab.wmv
- Report rights infringement
- published: 07 Sep 2010
- views: 5156

Understand the differences between Crystalline and Amorphous Solids
- Report rights infringement
- published: 08 Dec 2014
- views: 1738

Amorphous Solid Lab Day - 2.mp4
- Report rights infringement
- published: 08 Sep 2010
- views: 2015

Amorphous Solid Dispersion Formulations Using The Spray Dry Process
- Report rights infringement
- published: 19 Feb 2014
- views: 1872

Hot-Melt Extrusion Fundamentals: Processing of Amorphous Solid Dispersions for Poorly Soluble Drugs
- Report rights infringement
- published: 04 Nov 2015
- views: 318
- Playlist
- Chat

Hydrogenated Amorphous Silicon Cambridge Solid State Science Series
- Report rights infringement
- published: 30 Jun 2016
- views: 0

Difference Between Amorphous and Crystalline Solid
- Report rights infringement
- published: 07 May 2016
- views: 52

9th (Punjab Board) Chemistry Chapter 5.4 - LEC 3 Amorphous Solids
- Report rights infringement
- published: 04 May 2016
- views: 9

Define Solid | Types Of Waste | Amorphous Solid | Network Solid | Molecular Solids
- Report rights infringement
- published: 19 Apr 2016
- views: 8

Solid State NCERT
- Report rights infringement
- published: 12 Mar 2016
- views: 60

Amorphous solid
- Report rights infringement
- published: 22 Jan 2016
- views: 9

Amorphous solid - Video Learning - WizScience.com
- Report rights infringement
- published: 10 Sep 2015
- views: 81

Difference between Crystalline and Amorphous Solids
- Report rights infringement
- published: 24 Jun 2015
- views: 110
- Playlist
- Chat

How to : Crystalline and Amorphous Solid # Lecture # ClassXII
- Report rights infringement
- published: 27 Oct 2012
- views: 4526

Hot-Melt Extrusion of Amorphous Solid Dispersions for Bioavailability Enhancement
- Report rights infringement
- published: 24 Apr 2014
- views: 5234

13.1 Amorphous vs crystalline solids & classification of solids
- Report rights infringement
- published: 04 Dec 2013
- views: 3608

Lec 20 | MIT 3.091 Introduction to Solid State Chemistry
- Report rights infringement
- published: 29 Apr 2008
- views: 44046

Role of Excipients in Design of Solid Amorphous Dispersions - Thomas Durig
- Report rights infringement
- published: 07 Nov 2014
- views: 1337

Bravais Lattice ,Amorphous and Crystalline Solid in Solid State for IIT
- Report rights infringement
- published: 26 Jan 2013
- views: 5605

Plasticity and Material Failure in Amorphous Solids(Chandrasekhar Lecture II) by Itamar Procaccia
- Report rights infringement
- published: 11 May 2015
- views: 338

Lecture 20 Amorphous Solids, Glass Formation, Inorganic Glasses Silicates
- Report rights infringement
- published: 25 May 2014
- views: 380
'Maybe the Brits are just having us on': the world reacts to Boris Johnson as foreign minister
Edit The Guardian 14 Jul 2016Hillary and Jill – Jill Stein That Is – Went Up the Hill To…
Edit WorldNews.com 14 Jul 2016Are Obama and Clinton Counting on Republican Majorities to Pass TPP?
Edit CounterPunch 14 Jul 2016Trump Appears “Clueless” How He Could Win With This VP Pick
Edit WorldNews.com 13 Jul 2016Megachurch Pastor Says He'd Vote For Trump Over Jesus
Edit WorldNews.com 13 Jul 2016Local bodies told to prohibit discharge of sewage into river
Edit The Himalayan 14 Jul 2016Many trips, no solutions: Chandigarh councillors may head to Mysuru on latest study tour
Edit Indian Express 14 Jul 2016NGT asks Punjab, Haryana govt to furnish details on waste
Edit The Times of India 14 Jul 2016HELMA Eigenheimbau AG achieves new order growth of 7 percent to reach EUR 120.6 million in H1 2016 (HELMA Eigenheimbau AG)
Edit Public Technologies 14 Jul 2016Progressive (PGR) Stock Higher After Q2 Results
Edit The Street 14 Jul 2016JPMorgan's results beat forecasts as trading revenue jumps
Edit Springfield News-Sun 14 Jul 2016Elmassian makes solid start in Missouri (Golf Australia)
Edit Public Technologies 14 Jul 2016Press Release: Leave Inaccurate Concentrations Behind: Innovative Method Reduces Errors, Promotes Time and Cost Savings (Mettler Toledo International Inc)
Edit Public Technologies 14 Jul 2016US weekly claims for jobless benefits hold steady
Edit The Miami Herald 14 Jul 2016Govt allows SEZ units to import solid plastic waste to recycle
Edit Indian Express 14 Jul 2016Bobbili town: An inspiring lesson in smart waste management
Edit The Times of India 14 Jul 2016Southern Co. (SO) Stock Higher on Barclays Rating Upgrade
Edit The Street 14 Jul 2016What do Roman ceramics, earthquakes, catalysts and Matisse’s yellow have in common? Four young researchers explain their involvement in Future Energy & Resources (Universiteit Utrecht)
Edit Public Technologies 14 Jul 2016- 1
- 2
- 3
- 4
- 5
- Next page »







