Population deworming every 6 months with albendazole in 1 million pre-school children in north India: DEVTA, a cluster-randomised trial
Shally Awasthi, Richard Peto, [...], and the DEVTA (Deworming and Enhanced Vitamin A) team
Summary
Background
In north India many pre-school children are underweight, many have intestinal worms, and 2–3% die at ages 1·0–6·0 years. We used the state-wide Integrated Child Development Service (ICDS) infrastructure to help to assess any effects of regular deworming on mortality.
Methods
Participants in this cluster-randomised study were children in catchment areas of 8338 ICDS-staffed village child-care centres (under-5 population 1 million) in 72 administrative blocks. Groups of four neighbouring blocks were cluster-randomly allocated in Oxford between 6-monthly vitamin A (retinol capsule of 200 000 IU retinyl acetate in oil, to be cut and dripped into the child's mouth every 6 months), albendazole (400 mg tablet every 6 months), both, or neither (open control). Analyses of albendazole effects are by block (36 vs 36 clusters). The study spanned 5 calendar years, with 11 6-monthly mass-treatment days for all children then aged 6–72 months. Annually, one centre per block was randomly selected and visited by a study team 1–5 months after any trial deworming to sample faeces (for presence of worm eggs, reliably assessed only after mid-study), weigh children, and interview caregivers. Separately, all 8338 centres were visited every 6 months to monitor pre-school deaths (100 000 visits, 25 000 deaths at age 1·0–6·0 years [the primary outcome]). This trial is registered at ClinicalTrials.gov, NCT00222547.
Findings
Estimated compliance with 6-monthly albendazole was 86%. Among 2589 versus 2576 children surveyed during the second half of the study, nematode egg prevalence was 16% versus 36%, and most infection was light. After at least 2 years of treatment, weight at ages 3·0–6·0 years (standardised to age 4·0 years, 50% male) was 12·72 kg albendazole versus 12·68 kg control (difference 0·04 kg, 95% CI −0·14 to 0·21, p=0·66). Comparing the 36 albendazole-allocated versus 36 control blocks in analyses of the primary outcome, deaths per child-care centre at ages 1·0–6·0 years during the 5-year study were 3·00 (SE 0·07) albendazole versus 3·16 (SE 0·09) control, difference 0·16 (SE 0·11, mortality ratio 0·95, 95% CI 0·89 to 1·02, p=0·16), suggesting absolute risks of dying between ages 1·0 and 6·0 years of roughly 2·5% albendazole versus 2·6% control. No specific cause of death was significantly affected.
Interpretation
Existing ICDS village staff can be organised to deliver simple pre-school interventions sustainably for many years at low cost, but regular deworming had little effect on mortality in this lightly infected pre-school population.
Funding
UK Medical Research Council, USAID, World Bank (albendazole donated by GlaxoSmithKline).
Introduction
Heavy worm infection can constrain physical development.1,2 In areas where heavy infection is common, periodic deworming of school-age children can improve weight gain.3–5 Of three studies of urban pre-school children in Lucknow, north India, two suggested little effect of deworming on growth and one showed a substantial effect, perhaps large enough to reduce mortality significantly in a much larger study.6–9 The Integrated Child Development Service (ICDS) in Uttar Pradesh offered an opportunity to test the effects of deworming in children in rural areas.
The ICDS has staffed child-care centres serving all children up to age 6 years in one-third of the villages in Uttar Pradesh. These centres can deliver simple health interventions, as long as all the villages in the same administrative area are asked to give the same intervention.10,11 We therefore planned a large cluster-randomised trial of whether the ICDS could inexpensively and sustainably deliver anthelmintics at operational scale to rural pre-school children in Uttar Pradesh. The primary aim of the trial was to assess effects of a widely practicable periodic deworming regimen on mortality at ages 1·0–6·0 years.12
Plans for this deworming trial were eventually revised into plans for a factorial trial of albendazole, vitamin A (retinol), both, or neither that would assess the effects of each agent on child mortality. This report focuses on the albendazole results; an accompanying report gives the vitamin A results.13
Methods
Study design
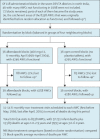
This cluster-randomised study spanned seven adjacent districts (area 35 000 km2, 3 degrees of latitude by 1 of longitude; figure 1). Excluding major municipalities, they consist of 118 largely rural administrative blocks (units that generally have a rural population of more than 100 000). 72 of these blocks could participate; 36 were cluster-randomly allocated albendazole every 6 months for 5 years and 36 open control (figure 2).
The study was based in Lucknow, the Uttar Pradesh state capital. The 72 participating blocks were those within a few hours drive that the ICDS director in Lucknow judged in 1998 to have a reasonably well functioning ICDS system with willing district and block ICDS directors and paid workers in most village anganwadi child-care centres (AWCs; anganwadi means courtyard). A typical rural AWC serves a village population of 1000 (10–15% aged 1–6·0 years); large villages can have more AWCs. The AWC workers, usually local women (plus assistants), give pre-school education, give nutritional supplements to malnourished children, and record births and pre-school deaths. AWCs register about two-thirds of under-5s (and one-third of 5-year-olds) for possible nutritional supplementation, although levels of AWC attendance and supplementary nutrition in Uttar Pradesh are often low.10 AWC workers can help health workers to organise immunisation and pre-school health services. In one block, a court order closed down all AWCs, but treatment and follow-up continued via the local administration (known as the panchayat).
In these 72 blocks, 8511 AWCs were deemed functional in 1998 (or, in a few cases, were expected to become functional during the study), and 8338 (98%) of these remained functional for most or all of the study and so were included (figure 2). At any one time the study population for assessment of mortality was all pre-school children then aged 1·0–6·0 years in the defined catchment areas of these 8338 AWCs. Reported age was often rounded up or down to approximate whole numbers of years.
In a typical block these study catchment areas included 10 000–20 000 children aged 1·0–6·0 years, so at any one time the study included 1 million such children. During the study another million 1-year-olds joined as the 6-year-olds left, so at one time or another the study included about 2 million children. The study continued for 5 years, and recorded 25 000 child deaths during 5 million child-years of follow-up. The study was approved by King George's Medical University ethics committee (Lucknow, India) and by the ICDS directors, but no permissions were obtained from the population, except when faecal samples were sought.
Randomisation and masking
Neighbouring blocks (clusters), in groups of four, were randomly allocated in Oxford, UK, using a factorial design to: (1) usual care; (2) 6-monthly vitamin A; (3) 6-monthly albendazole; or (4) both. No placebos were used; the control was open. Randomisation was stratified in groups of four blocks, where possible in the same district. No other relevant details of the blocks were known at baseline. This report compares albendazole (36 blocks) versus not (36 blocks), largely ignoring retinol allocation and stratum. Sensitivity analyses showed the main findings were unchanged when adjusted for district.
Procedures
GlaxoSmithKline donated albendazole in pots of 150 chewable tablets (400 mg Zentel). Dose was independent of age. When treatment became due (in April or October), in each treatment-allocated block a mass-treatment day was selected on which all children of apparent age 6–72 months in all study AWCs in that block would be given the trial treatment by their AWC worker. The few children missed were often treated soon afterwards.
Implementation of the mass-treatment days was encouraged and monitored independently by project staff and state government staff. At the previous monthly block meeting, each supervisor in the AWCs to be treated collected 150 tablets (plus extras, for large AWCs) and, on the selected day, administered treatment. Villagers were alerted beforehand.
To ensure AWC workers undertook mass-treatment on the chosen day, they were trained before the study (with refresher training in 45 blocks), discussed matters at their monthly block meetings before and afterwards, and knew that on all mass-treatment days unannounced visits were made to every fourth AWC on a numbered list for each block to check that distribution had started, followed by visits 1 week later to check numbers dispensed and interview focus groups about receiving treatment.
During the third study year, study monitors abstracted from local records name, sex, age, and father's name for all (just over 1 million) pre-school children in the study areas. There should have been little undercount of children, but numbers by month of age showed, as expected, substantial undercount of young infants, and ages were often approximate. After removal of apparently duplicated records (about 1% at ages 1·0–6·0 years), this mid-study census was used before each subsequent treatment day to list all in 25 AWCs per block with predicted age 12–70 months (ie, well within the range to be treated). Monitors established soon after whether the children listed had actually been treated (and whether they were currently registered with the AWC).
Annually, Oxford randomly selected one AWC per block for teams (one phlebotomist, two fieldworkers, one driver) to survey 1–5 months after a mass-treatment day 30 pre-school children (six per year of age; if fewer were available, a neighbouring AWC was included), seeking from them blood and faecal samples. In practice, full information with complete assay results was obtained for about 24 children per block. Children surveyed were not randomly chosen, and tended to be selected by AWC workers from those registered with the AWC. Villagers decided after public discussion whether the village would cooperate; if it did, no caregiver then chose to refuse consent. Only one selected village refused; an adjacent village replaced it.
After marking consent, caregivers were asked whether the previous mass-treatment had been received (and, if so, whether diarrhoea, vomiting, or fever had followed) and about recent (past 4 weeks) fast or difficult breathing suggesting WHO-defined pneumonia,14 diarrhoea (≥3 loose stools per day), measles, conjunctivitis, fever, and skin infection. Fieldwork teams were trained to measure height (to 1 mm; stadiometer), weight (to 0·1 kg; electronic scales, calibrated daily) and finger-prick haemoglobin.15 Blood and faecal samples were collected for central retinol assay16 and central formalin-ether-concentration helminth egg assay17 (useable only after mid-study, when egg-counting methodology greatly improved).
Deaths were recorded by 18 full-time motorcycle village-to-village monitors. Monitors covered four neighbouring blocks at a time, one in each treatment group, and within 6 months visited all (about 500) study AWCs in those blocks to identify and visit households where in the past year a liveborn infant or child younger than 10 years had died, recording age, sex, name, parental names, and (by simple verbal autopsy) likely cause.
Monitors were literate, but not medically trained (except by the study and experience during it). Identification of households where a death had occurred was from AWC workers, from their records, and from key informants (eg, village head, other village leaders, teacher, auxiliary nurse midwife). There was digit preference; ages were often rounded up or down, so we analyse deaths at age 1·0–6·0 years. Monitors knew supervisors would, with 5% probability, randomly re-study selected villages shortly afterwards to ensure the relevant households had all been visited (and to help consolidate monitors’ discipline and methods).
6 months later a different monitor visited the same blocks, again to record all deaths in the past year, so each death should have been reported twice, independently. Duplicates were eliminated manually, then by computer, in Lucknow, consulting monitors where necessary. The 238 remaining duplicates at ages 1·0–6·0 years were eliminated in Oxford by computer matches (199 with the same AWC, sex, first three letters of father's name, and date of death from a disease, 23 with matching for approximate date but closer other similarities, 16 with the same death reported in adjacent AWCs), leaving 25 582 child deaths for analysis. During the third study year, monitors further undertook house-to-house surveys in every AWC to identify any missed deaths.
Statistical analysis
Data were checked manually and (apart from our mid-study census) double-entered; any obvious errors were corrected in the primary records. The FoxPro database was sent monthly to Oxford and extensively queried, leading to interactive corrections. Faecal egg counts, anthropometry, blood assays, and recent morbidity rates were standardised (by multiple regression) for age, sex (50% male) and 6-month season (50% May–October: height and weight were further standardised for study half-year). Age standardisation was to 3·0, 2·0, or 4·0 years, respectively, for age ranges 1·0–6·0, 1·0–2·9, or 3·0–6·0 years.
The prespecified primary analysis (see appendix of accompanying report13) was of pre-school child mortality, operationalised because of digit preference in stating ages as mortality at reported ages 1·0–6·0 years during the 5-year study period. This outcome was secondarily subdivided into ages 1·0–2·9 and 3·0–6·0 years, and into study years 1–2 and 3–5. Analyses of the effects of albendazole allocation on any category of mortality (or on any other outcomes—eg, faecal egg counts, blood biochemistry, anthropometry, or morbidity) derived only from the 72 block averages for the outcome or explanatory factors of interest, giving all blocks equal weight (as they were of similar size). If albendazole did nothing, the mean of the 36 albendazole block averages would have been the mean of 36 selected randomly from these 72 values. Equivalently, the 72 values were regressed on two yes/no indicator values for retinol and albendazole allocation and on any explanatory factors.
Mortality analyses first calculated for each block the mean number of child deaths recorded per study AWC and the mean number of infant deaths recorded per study AWC. Analyses of effects on child mortality regressed child deaths per study AWC (72 values) on albendazole allocation (0/1), with simultaneous adjustment for retinol allocation (also 0/1), for an interaction term (±1, included only in simultaneous analyses of both treatments), and for infant deaths per AWC (an important explanatory factor that cannot have been materially affected by trial treatment, and varied two-fold between blocks). Sensitivity analyses explored the relevance of not adjusting for infant mortality or of additional adjustment for district (by which randomisation had been stratified). All these analyses did not depend on the mid-study census, but further sensitivity analyses explored the effect of adjustment for the census estimate of child population per AWC (an unimportant factor, since it varied little between blocks).
72 blocks were made available for this study by the ICDS. On the assumption that the 72 block-specific mortality rates observed in the study at ages 1·0–6·0 years would be about 2–3% with SD 0·5, the mean mortality reduction would have SE 0·12 (2SD/√72), so if treatment reduced mortality by 0·5% (ie, by about a fifth) the study would have a 95% chance of getting p<0·01. SAS version 9.1 was used. This trial is registered at ClinicalTrials.gov, NCT00222547.
Role of the funding source
Study sponsors had no role in design, conduct, analysis, or interpretation. SA, RP, DB, and SR had full data access and entirely controlled submission for publication.
Results
Compliance with treatment allocation was good. Unannounced visits to a quarter of the AWCs in the albendazole blocks on or just after each mass-treatment day confirmed that 98% (10 364/10 597 visits) were distributing treatment. Independently, enquiries a week after mass-treatment days about lists of named children from the mid-study census confirmed treatment of 95% (131 631/138 333) of those registered with the AWC and 73% (76 490/104 925) of those not. Because two-thirds of under-5s and one-third of 5-year-olds were AWC-registered, overall compliance was about 86%. Consistent with this finding, the biomedical surveys of randomly chosen AWCs, which tended to over-sample AWC-registered children, found caregivers reporting 92% (2388/2589) had received albendazole on the previous mass-treatment day. AWC workers in albendazole and control blocks held non-study mebendazole to treat children with obvious worms; otherwise, there was little non-study anthelmintic treatment. Loss to follow-up was similarly uncommon in both groups (2% [88/4246] albendazole, 2% [85/4265] control; figure 2), and was generally due to AWC closures.
Allocation to albendazole halved faecal worm egg prevalence 1–5 months after mass-treatment days. Faecal assays were available during the second half of the study for 5165 children with complete information on all assays and questionnaire replies, 2589 in albendazole-allocated versus 2576 in control blocks (table 1). Among them, the proportion with nematode (Ascaris or hookworm) eggs was 16% in the albendazole-allocated blocks versus 36% in control blocks, but in both albendazole and control groups any infection was generally light (median eggs per g faeces, if non-zero, Ascaris 96 [IQR 69–127], hookworm 83 [62–115]). The control prevalence matches previous estimates for north India.18,19 Trichuris eggs were not found. The proportion with tapeworm eggs (Hymenolepis spp) was, as expected, not significantly reduced.
Albendazole remains almost wholly inside the intestine, and oral treatment was not significantly associated with any immediate adverse effects. In 36 albendazole-allocated blocks, 362 children died during the 7 days before mass-treatment was due and 366 during the next 7 days. Caregivers were asked at the biomedical surveys whether the previous treatment had been closely followed by acute illness. No significant differences in vomiting, diarrhoea, or fever were seen between retinol plus albendazole, retinol alone, and albendazole alone. Among 6290 children in 18 retinol-and-albendazole blocks, vomiting was reported for 29 children in five blocks, diarrhoea for ten children in two blocks, and fever for six children in three blocks; among 6686 children in 18 albendazole-only blocks, vomiting was reported for 15 children in six blocks, diarrhoea for 12 in three blocks, and fever for three children in one block; among 6433 children in 18 retinol-only blocks, vomiting was reported for 25 children in four blocks, diarrhoea for 14 children in two blocks, and fever for 37 children in two blocks (both with obvious outbreaks).
Weight, height, and haemoglobin were not significantly improved by albendazole. After at least 2 years of treatment, mean weight at ages 3·0–6·0 years (standardised to age 4·0 years, half male, half female) in all surveyed children, infected or not, was 12·72 kg albendazole versus 12·68 kg control, difference 0·04 kg (95% CI −0·14 to 0·21 kg, p=0·66; table 2). This null result is statistically compatible with albendazole increasing weight by 0·5 kg in children who are actually infected. When, however, children in the same control village were compared with each other, the presence of faecal eggs was not associated with any significant differences in weight, height, or haemoglobin (table 2); the upper confidence limit for the weight difference associated with an individual having nematode eggs was only 0·2 kg. Likewise, there were no effects of allocation to albendazole on prevalence of ill health at the time of the visit (table 1). These null findings were independent of age and sex.
During the entire study, after exclusion of duplicated records and stillbirths, monitors recorded 97 231 deaths in infancy (86 084 before age 6 months), 15 589 at ages 1·0–2·9 years and 9993 at ages 3·0–6·0 years (figure 2). These numbers include any extra deaths identified by the special mid-study retrospective enquiry (which found similar numbers of missed child deaths in the two treatment groups, suggesting little ascertainment bias). Combined with our mid-study census listing a million children, this finding suggests at least 9% infant mortality and about 2·5% child mortality.
Figure 3 shows a 69% correlation between the numbers of infant and child deaths recorded per AWC. As any trial treatment in infancy began at 6–12 months of age and most infant deaths occur much earlier, overall infant mortality cannot have been materially affected by treatment. Hence, this strong correlation reflects differences not due to treatment between the numbers of infant deaths recorded per AWC. Confirming this conclusion, the correlation between infant and child mortality was equally strong among blocks that had all had the same treatment; figure 3. We therefore used number of infant deaths as an explanatory factor to reduce chance variation in our main analyses of the effects of treatment allocation on the number of child deaths.
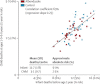
Table 3 shows the findings for child mortality at ages 1·0–2·9 years, 3·0–6·0 years, and 1·0–6·0 years, along with the relative risk for the age range 1·0–6·0 years. Overall child mortality was 5% lower in albendazole than in control blocks, but because randomisation was by block rather than by AWC or by individual this 5% difference is not significant (risk ratio [RR] 0·95, CI 0·88–1·02, p=0·16).
Sensitivity analyses (table 3) showed this RR was little changed (although its CI was narrowed) by adjustment for infant deaths, and would be little changed by use of 6-month mortality instead of infant mortality to correct for initial variation in prognosis, or by further adjustment for district or for mean number of children per AWC. There was no significant interaction between the effects of retinol and albendazole (interaction p=0·83). So, RR was again little changed (although its CI was widened) by restricting attention to 18 albendazole-plus-retinol versus 18 retinol-alone blocks, where monitoring could not be biased by one group getting no treatment. Table 3 also provides the separate results in all four (2×2) treatment groups.
The main contributor to the overall mortality difference was diarrhoeal mortality, which was 9% lower with albendazole, but this 9% difference was also not significant (RR 0·91, 95% CI 0·80–1·03). If the difference in overall mortality was a chance finding, the RR would be expected to be similar in various subgroups, and it was similar in the first 2 years and last 3 years (see accompanying report for illustration of the trial timelines13) and in boys and girls. There was an 80% correlation between male and female child mortality rates in different blocks.
The annual number of deaths per AWC was greater at ages 1·0–2·9 than at 3·0–6·0 years (particularly for diarrhoea, pneumonia, and malnutrition), greater in girls than in boys, and greater in earlier than in later study years, but for no category of age, disease, or sex was there a significant effect of albendazole on mortality. Even for children who had been on treatment more than 2 years (ie, for mortality at ages 3·0–6·0 years during the last 3 study years) there was no significant difference in mortality between blocks allocated albendazole and control.
To convert numbers of deaths per AWC into risks, population estimates are needed. Our mid-study census enumerated about 119 children of age 1·0–6·0 years per AWC (65 male, 54 female). Because of strong digit preferences in describing ages and possible undercount, particularly of young infants and children not registered with the AWC, this number is only approximate. Nevertheless, ignoring any uncertainties in enumeration, 11·7 infant deaths (6·2 male, 5·5 female) and 119 children per AWC suggests at least 9% infant mortality (11·7 / [11·7 + 119] plus some undercounted mortality in early infancy). Likewise, 3·1 child deaths per AWC (1·4 male, 1·6 female) suggests about 2·5% child mortality at ages 1·0–6·0 years (3·1/119). These infant and child mortality rates are consistent with published estimates for rural Uttar Pradesh.20,21
Discussion
Some but not all previous studies of periodic population deworming have suggested appreciable weight gain.1–3 The stated aims of the present albendazole trial were to establish whether twice-yearly deworming would be practicable within existing ICDS structures and, if so, whether it would provide sustainable benefits in terms of improved physical growth and (the primary outcome) survival of pre-school children.12 In terms of assessing effects on weight gain, this trial (which weighed only a representative few thousand children in a few dozen clusters) is no better powered than several previous studies, but in terms of assessing effects on survival it is unique (enumerating 25 000 child deaths).3,6–8,22,23 Because of digit preference in recording ages, the primary endpoint was operationalised as mortality at recorded ages 1·0–6·0 years. This outcome was secondarily subdivided into 1·0–2·9 and 3·0–6·0 years, and into the first 2 and last 3 study years. A major subsidiary aim was to establish whether the ICDS system could provide a cost-effective and sustainable delivery mechanism for this or other simple health interventions in pre-school children.
The subsidiary aim was fully achieved; 86% compliance was maintained over 5 years (with 95% compliance among the many children who were registered with the AWC). Focus group discussions attributed this compliance to political support, sustained and predictable drug supply, minimum additional work for AWC staff (who did not themselves have to report on compliance), helpful staff training meetings, and the knowledge that coverage might well be monitored. Excluding donated drug, the total cost was US$100 000 per year (US$0·10 per child), but this expense was mostly for evaluating the intervention. This suggests that, in villages with functioning ICDS anganwadi centres, delivery costs for simple pre-school interventions could be as low as for school-based health interventions.1,24,25
For the primary aim, however, despite good compliance for 5 years and despite halving the prevalence of worm infection, the randomised comparison did not provide significant evidence of an effect of deworming on survival (mortality RR 0·95, 95% CI 0·89–1·02). Although the confidence interval does not exclude a mortality reduction of about 10%, such an effect is unlikely since there was no significant increase in weight gain—the proxy for better nutrition, our postulated mechanism for mortality reduction.
The absence of a significant increase in weight is consistent with two previous studies of deworming this age group in north India,6,7 but discrepant with a third.8 These three previous studies were in urban areas in which the prevalence of Ascaris eggs among controls would have been about twice as great as in the present study.6 The upper confidence limit for the average weight gain in the second half of the present study was 0·2 kg, much less than the lower confidence limit of 0·7 kg for the 2-year gain in the third study.8 The weight for age of the children in these two studies was similar, suggesting similar nutritional status, and we led both trials and are confident of the reliability of each. This substantial discrepancy between the apparent effects on weight gain in the two studies remains unexplained.
Previous studies of population mortality from soil-transmitted helminths have involved estimates of mortality from communities or hospital populations in unusual circumstances and offer little guidance as to the direct and indirect effects in large general populations.26–29 Although further studies of the indirect effects on mortality in much more intensely infected or worse nourished populations could well be worthwhile, reductions in mortality might be difficult to demonstrate because of the age distribution of infection; for example, although the intensity of infection might be much greater in older child populations, they have lower overall mortality rates.1
Overall, the present study shows that in this lightly infected rural population routine deworming of pre-school children had little effect on mortality (panel). This finding does not rule out effects on mortality in other populations, but any such effects are generally likely to be small, so reduction in population mortality is unlikely to be a primary aim of deworming programmes.30
Acknowledgments
This report is dedicated to its onlie begetter, Kenneth S Warren (1929–96). Our chief acknowledgment is to the AWC workers, communities, and children in the 8338 participating AWCs; the seven district and 72 ICDS officials in the following districts and blocks (Lucknow: Mall, Malihabad, Bakshi Ka Talab, Chinhat, Sarojini Nagar, Kakori, Mohan Lal Ganj, Gosai Ganj; Unnao: Sikandarpur Karan, Sikandarpur Sirosi, Hasan Ganj, Miyan Ganj, Asoha, Auras, Safipur, Bangarmau, Fatehpur Chourasi, Bichhiya, Sumerpur, Bighapur, Purva, Ganjmuradabad; Kanpur: Kanpur Nagar I, Kanpur Nagar II, Sarsoul, Bilhour, Patara, Vidhnu, Choubepur, Kalyanpur; Sitapur: Mishrikh, Sakaran, Khairabad, Godlamau, Hargaon, Kasmanda, Machrehta, Biswan, Pahla; Lakhimpur Kheeri: Lakhimpur Kheeri, Nidyasan, Vijuva, Ishanagar, Dharora, Pasgawan, Mohammadi, Golagokaran Nath, Bakey Ganj, Mitouli, Paliya Kala, Nakha, Phool Behad, Behjam; Raibareli: Singhpur, Dalmau, Unchahar, Maharajganj, Bahadurpur, Salon, Harchanpur, Bachrawana, Khero, Tiloi; Hardoi: Kachhouna, Kothawa, Bilgram, Hariyawan, Pihani, Tadiawan, Sursa, Ahirori, Behinder); the Government of Uttar Pradesh Department of Health and Department of Woman and Child Welfare (responsible for the ICDS); and the Vice-Chancellor of King George's Medical University, M Bhandari. Rajiv Awasthi, Jill Boreham, Frances Davidson, Nilanthi de Silva, Martin Frigg (1943–2010), Andrew Hall, John Horton, Trudie Lang, Penny Nestel, Lorenzo Savioli, and Sarman Singh helped to plan, execute, or comment on the study. Funding was received from the USAID OMNI project, World Bank, and UK Medical Research Council (via CTSU). Albendazole (Zentel) was donated by SmithKlineBeecham (now part of GlaxoSmithKline), and vitamin A by Roche vitamins (now part of DSM) via the Sight and Life Program. We acknowledge the CTSU's computing and other support in Oxford, including funds from the 1991 Helmut Horten cancer research award to Richard Peto and Richard Doll (1912–2005).
Contributors
SA, RP, and DB contributed to the design. SA and VP were responsible for organisation and conduct. SR and SMR undertook data management. RP and SR were responsible for the analysis. RP, SR, and DB drafted the report. All authors contributed to redrafting.
DEVTA staff
Computing Lakshmi Ayyar, Atul Chandra, Vipin Chandra Joshi, Nisha Narang, Hasibur Rehman, Nikhil Saxena, Naveen Prakash, Anuradha Sharma, Monika Sharma, Manish Tripathi, Deepak Kumar Upreti; Documentation Rohini Das, Anupama Lal, Tuhina Rastogi; Finance and accounts S S Mani, Dheeraj Chitransh, Lalit Pandey, Amit Tandon; Laboratory Durgesh Bajpai, Umesh Chandra, Arunesh Dwivedi, P K Pant, Vivekanand Shukla; Motorcycle monitors Amir Ahmad, Hafeez Ahmad, Jitendra Bahadur, Harish Chandra, Ramesh Chandra, Anil Chaturvedi, Shailesh Dwivedi, Amar Kumar, Digant Kumar, Mahesh Kumar, Neelu Kumar, Rajesh Kumar, Sudheer Kumar, Sunil Kumar I (who died while working for the study), Sunil Kumar II, Chandra Pal, Mahesh Prasad, Rakesh Kumar I, Rakesh Kumar II, Zafar Rashid, Hitler Singh, Hanslal Shukla, Kunj Bihari Shukla, Shiv Shanker Shukla, Vipin Bihari Shukla, Sangram Singh, Satyawan Singh, Shiv Shankar Verma, Shiv Singh Verma; Drivers Ramesh Chandra, Inamul Haq, Mohammed Kazi, Ajay Kumar, Keshav Kumar, Manoj Kumar, Sharawan Kumar, Bansi Lal, Ashok Kumar Tiwari; Office and fieldwork management Shrawan Awasthi, Alpana Maseeh, Safia Najeeb, Lalji Neetu, Shahnaaz Parween, B Rai, Tuhina Rastogi, Ajay Sharma, Reetu Shukla, Sudha Shukla, Lalji Shukla, Anuj Srivastava, Vinay Kumar Srivastava.
Conflicts of interest
We declare that we have no conflicts of interest.