Tweet
- Tweet, halaman saat ini.
- Tweet & balasan
- Media
Anda memblokir @FDATobacco
Yakin ingin melihat Tweet ini? Melihat Tweet tidak akan membuka blokir @FDATobacco
-
Tweet Sematan
Today, FDA announced it has authorized the marketing of three new tobacco products, marking the first set of electronic nicotine delivery system (ENDS) products ever authorized by FDA through the Premarket Tobacco Product Application (PMTA) pathway. https://www.fda.gov/news-events/press-announcements/fda-permits-marketing-e-cigarette-products-marking-first-authorization-its-kind-agency?utm_source=CTPTwitter&utm_medium=social&utm_campaign=ctp-pmta …pic.twitter.com/KUd8zHb1l6
 Tampilkan utas iniTerima kasih. Twitter akan menggunakan ini untuk membuat timeline Anda lebih baik. BatalkanBatalkan
Tampilkan utas iniTerima kasih. Twitter akan menggunakan ini untuk membuat timeline Anda lebih baik. BatalkanBatalkan -
How can an NRT help me quit? FDA-approved nicotine replacement therapies (NRTs) help adults quit smoking by delivering small amounts of nicotine to the brain without the toxic mix of chemicals found in cigarette smoke. Learn more here: https://go.usa.gov/xM6tZ pic.twitter.com/kZ1ioupyP5
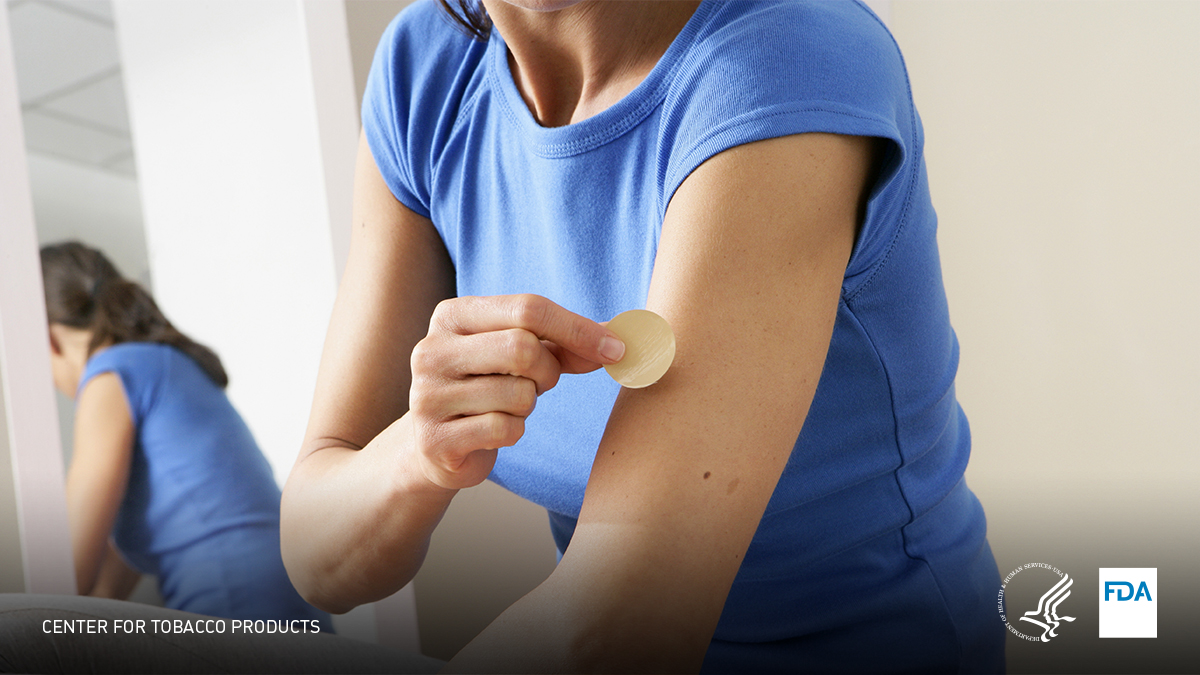 Terima kasih. Twitter akan menggunakan ini untuk membuat timeline Anda lebih baik. BatalkanBatalkan
Terima kasih. Twitter akan menggunakan ini untuk membuat timeline Anda lebih baik. BatalkanBatalkan -
Secondhand smoke causes more than 40,000 deaths a year. Help protect your loved ones’ health by becoming and staying smoke-free. Make your quit plan today at https://smokefree.gov .pic.twitter.com/DE1RTHbbbB
 Terima kasih. Twitter akan menggunakan ini untuk membuat timeline Anda lebih baik. BatalkanBatalkan
Terima kasih. Twitter akan menggunakan ini untuk membuat timeline Anda lebih baik. BatalkanBatalkan -
FDA Tobacco me-Retweet
In 2021, more than 8 in 10 U.S. youth who currently used e-cigarettes used a flavored product. Fruit flavors were the most commonly used. Get more of the latest data on youth e-cigarette use in
@CDCMMWR: http://bit.ly/MMWR92921 pic.twitter.com/833YWiLuun Terima kasih. Twitter akan menggunakan ini untuk membuat timeline Anda lebih baik. BatalkanBatalkan
Terima kasih. Twitter akan menggunakan ini untuk membuat timeline Anda lebih baik. BatalkanBatalkan -
Do you educate teens on the dangers of vaping? FDA developed youth e-cigarette prevention posters to help your efforts. Order or download now for free. https://digitalmedia.hhs.gov/tobacco/print_materials/search?audience=Youth&page=1&tag=E-Cigarettes+%26+Vaping&utm_source=CTPTwitter&utm_medium=social&utm_campaign=ctp-exchangelab …pic.twitter.com/ZA9wumg7e1
 Terima kasih. Twitter akan menggunakan ini untuk membuat timeline Anda lebih baik. BatalkanBatalkan
Terima kasih. Twitter akan menggunakan ini untuk membuat timeline Anda lebih baik. BatalkanBatalkan -
FDA wants to know about tobacco-related health or quality problems. Report adverse experiences to the Safety Reporting Portal: https://www.safetyreporting.hhs.gov
Terima kasih. Twitter akan menggunakan ini untuk membuat timeline Anda lebih baik. BatalkanBatalkan -
FDA Tobacco me-Retweet
Infants exposed to secondhand smoke are more likely to die of sudden infant death syndrome (SIDS). This
#SIDSAwarenessMonth, quit and make your home smoke-free for those you love most.pic.twitter.com/d0GMPaBVwr Terima kasih. Twitter akan menggunakan ini untuk membuat timeline Anda lebih baik. BatalkanBatalkan
Terima kasih. Twitter akan menggunakan ini untuk membuat timeline Anda lebih baik. BatalkanBatalkan -
“This is Our Watch” digital age verification calendars help retailers quickly determine if a customer is old enough to legally purchase tobacco products. Under federal law, the minimum age of tobacco purchase is 21. These calendars are free to order. https://digitalmedia.hhs.gov/tobacco/print_materials/re-26?utm_source=CTPTwitter&utm_medium=social&utm_campaign=ctp-exchangelab …pic.twitter.com/qlY7bYHPe9
 Terima kasih. Twitter akan menggunakan ini untuk membuat timeline Anda lebih baik. BatalkanBatalkan
Terima kasih. Twitter akan menggunakan ini untuk membuat timeline Anda lebih baik. BatalkanBatalkan -
ICYMI: A report released from
@FDATobacco and@CDCGov shows >2 mil. U.S. middle and high school students reported currently using e-cigarettes in 2021, with >8 in 10 using flavored e-cigarettes. The report was based on data from the 2021 NYTS. https://go.usa.gov/xMHJg pic.twitter.com/yDEfTLG9Nz Terima kasih. Twitter akan menggunakan ini untuk membuat timeline Anda lebih baik. BatalkanBatalkan
Terima kasih. Twitter akan menggunakan ini untuk membuat timeline Anda lebih baik. BatalkanBatalkan -
Sign up for CTP email updates, including email alerts when MRTP application materials are published.https://www.fda.gov/tobacco-products/ctp-newsroom/sign-email-updates-ctp?utm_source=CTPTwitter&utm_medium=social&utm_campaign=ctp-newsletter …
Terima kasih. Twitter akan menggunakan ini untuk membuat timeline Anda lebih baik. BatalkanBatalkan -
More than 8 out of 10 lung cancer deaths in the U.S. can be attributed to smoking. This Healthy Lung Month, start your journey to quit smoking. Visit https://smokefree.gov for free tips and resources to help you get and stay smoke-free.pic.twitter.com/aG8bxVl00x
Terima kasih. Twitter akan menggunakan ini untuk membuat timeline Anda lebih baik. BatalkanBatalkan -
Additionally, the webinar will highlight the new Tobacco Registration & Product Listing Module Next Generation, which manufacturers can use to submit the required information to the FDA. To watch more FDA Tobacco Compliance Webinars, visit:https://www.fda.gov/tobacco-products/compliance-enforcement-training/fda-tobacco-compliance-webinars?utm_source=CTPTwitter&utm_medium=social&utm_campaign=ctp-webinars …
Tampilkan utas iniTerima kasih. Twitter akan menggunakan ini untuk membuat timeline Anda lebih baik. BatalkanBatalkan -
This webinar provides a brief overview of section 905 of the Federal Food, Drug, and Cosmetic Act (FD&C Act), which describes the establishment registration and product listing requirements for Tobacco Product manufacturers.
Tampilkan utas iniTerima kasih. Twitter akan menggunakan ini untuk membuat timeline Anda lebih baik. BatalkanBatalkan -
FDA’s Center for Tobacco Products invites you to watch a new Tobacco Compliance Webinar, “Tobacco Registration and Product Listing Updates.”https://www.youtube.com/watch?v=cSbHQ-beIHY …
Tampilkan utas iniTerima kasih. Twitter akan menggunakan ini untuk membuat timeline Anda lebih baik. BatalkanBatalkan -
FDA Tobacco me-Retweet
Today, FDA announced it has authorized the marketing of three new tobacco products, marking the first set of electronic nicotine delivery system (ENDS) products ever authorized by FDA through the Premarket Tobacco Product Application (PMTA) pathway. https://www.fda.gov/news-events/press-announcements/fda-permits-marketing-e-cigarette-products-marking-first-authorization-its-kind-agency?utm_source=CTPTwitter&utm_medium=social&utm_campaign=ctp-pmta …pic.twitter.com/KUd8zHb1l6
 Tampilkan utas iniTerima kasih. Twitter akan menggunakan ini untuk membuat timeline Anda lebih baik. BatalkanBatalkan
Tampilkan utas iniTerima kasih. Twitter akan menggunakan ini untuk membuat timeline Anda lebih baik. BatalkanBatalkan -
FDA Tobacco me-Retweet
This is an important step toward ensuring all new tobacco products undergo FDA’s scientific premarket evaluation. Under the PMTA pathway, manufacturers must demonstrate that marketing of the new tobacco product would be appropriate for the protection of the public health.https://twitter.com/FDATobacco/status/1448010666019852295 …
 Terima kasih. Twitter akan menggunakan ini untuk membuat timeline Anda lebih baik. BatalkanBatalkan
Terima kasih. Twitter akan menggunakan ini untuk membuat timeline Anda lebih baik. BatalkanBatalkan -
FDA also issued 10 Marketing Denial Orders (MDOs) for flavored ENDS products submitted under the Vuse Solo brand by RJR. These products subject to an MDO for a premarket application may not be introduced or delivered for introduction into interstate commerce.
Tampilkan utas iniTerima kasih. Twitter akan menggunakan ini untuk membuat timeline Anda lebih baik. BatalkanBatalkan -
While today’s action permits the tobacco products to be sold in the U.S., it does not mean these products are safe or “FDA approved.” All tobacco products are harmful and addictive and those who do not use tobacco products should not start.
Tampilkan utas iniTerima kasih. Twitter akan menggunakan ini untuk membuat timeline Anda lebih baik. BatalkanBatalkan -
As the RJR Vapor Company submitted data to FDA that demonstrated that marketing of these products is appropriate for the protection of public health, today’s authorization allows these products to be legally sold in the U.S.
Tampilkan utas iniTerima kasih. Twitter akan menggunakan ini untuk membuat timeline Anda lebih baik. BatalkanBatalkan -
FDA issued Marketing Granted Orders to R.J. Reynolds Vapor Company for its Vuse Solo closed ENDS device & accompanying tobacco-flavored e-liquid pods, specifically, Vuse Solo Power Unit, Vuse Replacement Cartridge Original 4.8% G1 & Vuse Replacement Cartridge Original 4.8% G2.
Tampilkan utas iniTerima kasih. Twitter akan menggunakan ini untuk membuat timeline Anda lebih baik. BatalkanBatalkan
Pemuatan tampaknya berlangsung agak lama.
Twitter sedang kelebihan beban atau mengalami sedikit masalah. Coba lagi atau kunjungi Status Twitter untuk informasi lebih lanjut.




