Analysis of US Food and Drug Administration Warning Letters: False Promotional Claims Relating to Prescription and Over-the-Counter Medications
- PMID: 24353430
- PMCID: PMC3864040
- DOI: 10.1007/BF03256691
Analysis of US Food and Drug Administration Warning Letters: False Promotional Claims Relating to Prescription and Over-the-Counter Medications
Abstract
Background: Recent studies have suggested that there has been an increase in the number of 'warning letters' issued by the US Food and Drug Administration (FDA) despite the publication of the FDA advertising guidelines. However, limited information is available on the description of warning letters. The objective of this study was to analyse the frequency and content of FDA warning letters in relation to promotional claims and discuss the influence of regulatory and industry constraints on promotion.
Methods: All warning letters published by the FDA between 5 May 1995 and 11 June 2007 were reviewed. Warning letters related to promotional issues were included and analysed. Information related to the identification number, date of the warning letter, FDA division that issued the letter, drug name, manufacturer, specific warning problem, type of promotional material and requested action was extracted. Two independent investigators reviewed and classified each PDF file, any differences were discussed until a consensus was reached.
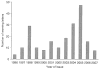
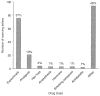
Results: Between May 1995 and June 2007 a total of 8692 warning letters were issued, of which 25% were related to drugs. Of these, 206 warning letters focused on drug promotion and were included in this study: 23% were issued in 2005, 15% in 2004 and 14% in 1998. In total, 47% of the warning letters were issued because of false or misleading unapproved doses and uses, 27% failed to disclose risks, 15% cited misleading promotion, 8% related to misleading labelling and 3% promoted false effectiveness claims.
Discussion: There is an important variation in the number of warning letters issued in the last decade, probably because of the increasing number of drugs approved by the FDA, drug withdrawal scandals, and the publication of the FDA and the Pharmaceutical Research and Manufacturers of America (PhRMA) guidelines.
Conclusion: We found that benefit-related claims, such as unapproved uses or doses of drugs, and failure to disclose risks, are the main causes of FDA issued warning letters for promotional claims related to medications.
Conflict of interest statement
The authors have no conflicts of interest that are directly relevant to the content of this study.
Figures


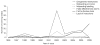

Similar articles
-
An analysis of the warning letters issued by the FDA to pharmaceutical manufacturers regarding misleading health outcomes claims.Pharm Pract (Granada). 2012 Oct;10(4):194-8. doi: 10.4321/s1886-36552012000400003. Epub 2012 Dec 31. Pharm Pract (Granada). 2012. PMID: 24155837 Free PMC article.
-
FDA actions against misleading or unsubstantiated economic and quality-of-life promotional claims: an analysis of warning letters and notices of violation.Value Health. 2002 Sep-Oct;5(5):390-7. doi: 10.1046/J.1524-4733.2002.55146.x. Value Health. 2002. PMID: 12201856
-
Health Claims About Cannabidiol Products: A Retrospective Analysis of U.S. Food and Drug Administration Warning Letters from 2015 to 2019.Cannabis Cannabinoid Res. 2021 Dec;6(6):559-563. doi: 10.1089/can.2020.0166. Epub 2021 Jun 17. Cannabis Cannabinoid Res. 2021. PMID: 34142863
-
Content Analysis of 2012-2019 FDA Warning Letters and Notices of Violations using the Economic, Clinical, and Humanistic Outcomes (ECHO) Model.Innov Pharm. 2021 Jan 13;12(1):10.24926/iip.v12i1.3420. doi: 10.24926/iip.v12i1.3420. eCollection 2021. Innov Pharm. 2021. PMID: 34007685 Free PMC article.
-
Prescription of Controlled Substances: Benefits and Risks.2021 Aug 31. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan–. 2021 Aug 31. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan–. PMID: 30726003 Free Books & Documents. Review.
Cited by 2 articles
-
Content analysis of false and misleading claims in television advertising for prescription and nonprescription drugs.J Gen Intern Med. 2014 Jan;29(1):110-8. doi: 10.1007/s11606-013-2604-0. J Gen Intern Med. 2014. PMID: 24030427 Free PMC article.
-
Risk Communication and the Pharmaceutical Industry: what is the reality?Drug Saf. 2012 Nov 1;35(11):1027-40. doi: 10.1007/BF03261989. Drug Saf. 2012. PMID: 23061779
Grant support
LinkOut - more resources
Full Text Sources
