Latest News for: alpha helix
Edit
 The Citizens
31 Jul 2021
The Citizens
31 Jul 2021
Five takeaways on the science behind CDC's latest mask guidance
 The Citizens
31 Jul 2021
The Citizens
31 Jul 2021
In the United States and around the world, it swiftly overtook another variant, Alpha, that was already more transmissible than earlier strains. New research from Helix, a company whose Covid-19 tests have helped track a number of variants, shows how quickly that happened. Alpha made up 67% of cases in mid-May.
Edit
 The Keene Sentinel
28 Jul 2021
The Keene Sentinel
28 Jul 2021
CDC urges vaccinated people in COVID hot spots to resume wearing masks indoors
 The Keene Sentinel
28 Jul 2021
The Keene Sentinel
28 Jul 2021
officials by surprise ... Meanwhile, the alpha variant, first seen in the United Kingdom, became the dominant strain across the country. But in June, the delta variant began to spread at exponential rates, said scientist William Lee of the genomics company Helix ... The alpha variant, by contrast, is seen in only about 3 percent of positive tests ... .
Edit
The highly infectious Delta variant may already be dominant in the US, says leading coronavirus ...
Business Insider 30 Jun 2021
Edit
 The Citizens
30 Jun 2021
The Citizens
30 Jun 2021
Rise of Delta variant brings mask question back, even for the vaccinated
 The Citizens
30 Jun 2021
The Citizens
30 Jun 2021
Mulligan predicted that would rise to 40% soon, and the genetic sequencing company Helix said its testing indicates Delta already accounts for 40% of cases. The B.1.1.7 or Alpha lineage of the virus, first spotted in the UK, was still the dominant variant in the US as of June 19, representing 47.8% of cases, the CDC estimated.
Edit
New deadly delta variant of coronavirus gains steam in undervaccinated U.S. counties
Crains Detroit Business 25 Jun 2021
The genomics firm Helix analyzed about 20,000 samples from COVID-19 tests across more than 700 U.S ... "Delta is driving surges around the world, and I suspect it's going to be the same here," said William Lee, the vice president of science at Helix ... But the "gamma and delta have gained a strong foothold and may soon push alpha out," Helix's Lee said.
Edit
Experimental prostate cancer drug may improve COVID-19 survival; younger patients report long lasting symptoms
Gulf Daily News 24 Jun 2021
Edit
Delta variant gains steam in undervaccinated US counties
Indian Express 23 Jun 2021
The genomics firm Helix analyzed about 20,000 samples from Covid-19 tests across more than 700 US counties ... The Helix research, which hasn’t yet been subject to peer review, is to be published in an upcoming pre-print online ... But the “gamma and delta have gained a strong foothold and may soon push alpha out,” Helix’s Lee said.
Edit
Covid-19 Delta variant gains steam in undervaccinated US counties
Hindustan Times 22 Jun 2021
The genomics firm Helix analyzed about 20,000 samples from Covid-19 tests across more than 700 US counties ... The alpha variant, first found in the U.K., has been the dominant strain in the US But the “gamma and delta have gained a strong foothold and may soon push alpha out,” Helix’s Lee said.
Edit
Delta Covid-19 variant seen spreading in under-vaccinated US counties
Straits Times 22 Jun 2021
The genomics firm Helix analysed about 20,000 samples from Covid-19 tests across more than 700 US counties ... The Helix research, which hasn't yet been subject to peer review, is to be published in an upcoming pre-print online ... But the "gamma and delta have gained a strong foothold and may soon push alpha out," Helix's Lee said.
Edit
Delta COVID variant seen spreading in undervaccinated US counties
Live Mint 22 Jun 2021
The genomics firm Helix analyzed about 20,000 samples from Covid-19 tests across more than 700 U.S ... “Delta is driving surges around the world, and I suspect it’s going to be the same here," said William Lee, the vice president of science at Helix ... But the “gamma and delta have gained a strong foothold and may soon push alpha out," Helix’s Lee said.
Edit
We went inside the labs where DNA-testing startup Helix has been running coronavirus tests
Business Insider 15 Jun 2021
Edit
Impact of an alpha helix and a cysteine-cysteine disulfide bond on the resistance of bacterial ...
PNAS 19 May 2021
Edit
DNA-inspired 'supercoiling' fibers could make powerful artificial muscles for robots
Phys Dot Org 29 Apr 2021
The double helix of DNA is one of the most iconic symbols in science ... The power of the helix. DNA is not the only helix in nature. Flip through any biology textbook and you'll see helices everywhere from the alpha-helix shapes of individual proteins to the "coiled coil" helices of fibrous protein assemblies like keratin in hair.
Edit
Oligourea foldamers mimic peptides' alpha-helices and effectively bind to drug targets
Phys Dot Org 07 Dec 2020
These oligoureas fold into a helix, one of the hallmark structures of peptides ... "Oligourea helices have fewer residues per turn, a smaller rise per turn, and a larger diameter than the original peptide alpha-helix," says Guichard ... "Alpha amino acids can be substituted at two positions, but ureido units have one site more," says Guichard.
- 1
- 2
- Next page »


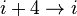 hydrogen bonding). This secondary structure is also sometimes called a classic Pauling–Corey–Branson alpha helix (see below). The name 3.613-helix is also used for this type of helix, denoting the number of residues per helical turn, and 13 atoms being involved in the ring formed by the hydrogen bond. Among types of local structure in proteins, the α-helix is the most regular and the most predictable from sequence, as well as the most prevalent.
hydrogen bonding). This secondary structure is also sometimes called a classic Pauling–Corey–Branson alpha helix (see below). The name 3.613-helix is also used for this type of helix, denoting the number of residues per helical turn, and 13 atoms being involved in the ring formed by the hydrogen bond. Among types of local structure in proteins, the α-helix is the most regular and the most predictable from sequence, as well as the most prevalent.














