- published: 14 Sep 2014
- views: 372696
-
remove the playlistProtactinium
- remove the playlistProtactinium
- published: 04 Nov 2018
- views: 501
- published: 18 Oct 2017
- views: 6863
- published: 10 Sep 2015
- views: 1452
- published: 11 Apr 2019
- views: 454698
- published: 05 Dec 2009
- views: 18328

Protactinium
Protactinium is a chemical element with symbol Pa and atomic number 91. It is a dense, silvery-gray metal which readily reacts with oxygen, water vapor and inorganic acids. It forms various chemical compounds where protactinium is usually present in the oxidation state +5, but can also assume +4 and even +2 or +3 states. The average concentrations of protactinium in the Earth's crust is typically on the order of a few parts per trillion, but may reach up to a few parts per million in some uraninite ore deposits. Because of its scarcity, high radioactivity and high toxicity, there are currently no uses for protactinium outside of scientific research, and for this purpose, protactinium is mostly extracted from spent nuclear fuel.
Protactinium was first identified in 1913 by Kasimir Fajans and Oswald Helmuth Göhring and named brevium because of the short half-life of the specific isotope studied, namely protactinium-234. A more stable isotope (231Pa) of protactinium was discovered in 1917/18 by Otto Hahn and Lise Meitner, and they chose the name proto-actinium, but then the IUPAC named it finally protactinium in 1949 and confirmed Hahn and Meitner as discoverers. The new name meant "parent of actinium" and reflected the fact that actinium is a product of radioactive decay of protactinium. It is noted that John Arnold Cranston (working with Frederick Soddy) is also credited with discovering the stable isotope in 1915 but delayed his announcement due to being called up for service in the First World War.
Isotopes of protactinium
Protactinium (Pa) has no stable isotopes. There are three naturally occurring isotopes, allowing a standard mass to be given.
Relative atomic mass: 231.03588(2).
Twenty-nine radioisotopes of protactinium have been characterized, with the most stable being 231Pa with a half-life of 32,760 years, 233Pa with a half-life of 26.967 days, and 230Pa with a half-life of 17.4 days. All of the remaining radioactive isotopes have half-lives that are less than 1.6 days, and the majority of these have half-lives that are less than 1.8 seconds. This element also has 3 meta states, 217mPa (t1/2 1.15 milliseconds), 229mPa (t1/2 420 nanoseconds), and 234mPa (t1/2 1.17 minutes).
The only naturally occurring isotopes are 231Pa, which occurs as an intermediate decay product of 235U, 234Pa and 234mPa, both of which occur as intermediate decay products of 238U. 231Pa makes up nearly all natural protactinium.
The primary decay mode for isotopes of Pa lighter than (and including) the most stable isotope 231Pa is alpha decay, except for 228Pa to 230Pa, which primarily decay by electron capture to isotopes of thorium. The primary mode for the heavier isotopes is beta minus (β−) decay. The primary decay products of 231Pa and isotopes of protactinium lighter than and including 227Pa are isotopes of actinium and the primary decay products for the heavier isotopes of protactinium are isotopes of uranium.
-

Protactinium - Periodic Table of Videos
Here's a new video about Protactinium, number 91 on the periodic table! Videos on all 118 elements: http://bit.ly/118elements Eric Scerri's book (UK): http://bit.ly/SevenScerriUK And (US): http://bit.ly/SevenScerriUS (*) Isotope 238 has half life 6.8 hrs Isotope 238m has half life 1.17 mins More chemistry at http://www.periodicvideos.com/ Follow us on Facebook at http://www.facebook.com/periodicvideos And on Twitter at http://twitter.com/periodicvideos From the School of Chemistry at The University of Nottingham: http://bit.ly/NottChem Periodic Videos films are by video journalist Brady Haran: http://www.bradyharan.com/ A run-down of Brady's channels: http://bit.ly/bradychannels
published: 14 Sep 2014 -
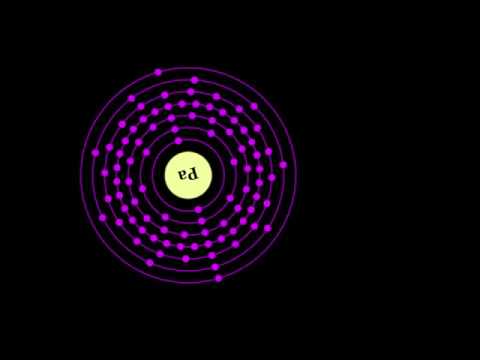
What is PROTACTINIUM?
What is PROTACTINIUM? Protactinium formerly known as protoactinium is a chemical element with symbol Pa and atomic number 91. It is a dense, silvery-gray actinide metal which readily reacts with oxygen, water vapor and inorganic acids. It forms various chemical compounds in which protactinium is usually present in the oxidation state +5, but it can also assume +4 and even +3 or +2 states. Concentrations of protactinium in the Earth's crust are typically a few parts per trillion, but may reach up to a few parts per million in some uraninite ore deposits. Because of its scarcity, high radioactivity and high toxicity, there are currently no uses for protactinium outside scientific research, and for this purpose, protactinium is mostly extracted from spent nuclear fuel. Protactinium was fir...
published: 04 Nov 2018 -

Protactinium half life experiment
The background count can be determined from this video https://www.youtube.com/watch?v=nTHfO8daPZc Using a protactinium genrator and a gieger-muller tube, you can perform an experiment to determine the half life of a protactinium isotope. This video explains how the protactinium generator works and will allow you to take results in real time that will allow you to determine the decay constant and the half-life.
published: 18 Oct 2017 -

Protactinium - Video Learning - WizScience.com
"Protactinium" is a chemical element with symbol "Pa" and atomic number 91. It is a dense, silvery-gray metal which readily reacts with oxygen, water vapor and inorganic acids. It forms various chemical compounds where protactinium is usually present in the oxidation state +5, but can also assume +4 and even +2 or +3 states. The average concentrations of protactinium in the Earth's crust is typically on the order of a few parts per trillion, but may reach up to a few parts per million in some uraninite ore deposits. Because of its scarcity, high radioactivity and high toxicity, there are currently no uses for protactinium outside of scientific research, and for this purpose, protactinium is mostly extracted from spent nuclear fuel. Protactinium was first identified in 1913 by Kasimir Fa...
published: 10 Sep 2015 -

Come Around
Come around by Protactinium
published: 29 Sep 2020 -

Incredibly rare and radioactive elements ☢
Plutonium, Uranium, Holmium, Neptunium, Curium and many more. We're at the Oak Ridge National Laboratory, in Tennessee. More links and info in full description ↓↓↓ ORNL: https://www.ornl.gov Our thanks to Rose Boll and the team. Our videos on all 118 elements: http://bit.ly/118elements Our previous video from Oak Ridge (the Thorium Cow): https://youtu.be/DmczVhGq8cU Our Dubna visit: http://bit.ly/Russia_Trip This video features isotopes of Plutonium, Uranium, Neptunium, Americium, Protactinium, Actinium, Technetium, Berkelium, Holmium, Californium, Curium and Polonium. Support us on Patreon: https://www.patreon.com/periodicvideos More chemistry at http://www.periodicvideos.com/ Follow us on Facebook at http://www.facebook.com/periodicvideos And on Twitter at http://twitter.com/peri...
published: 11 Apr 2019 -

The Half-life of Protactinium
The decay of Protactinium (from a bottled source) is monitored using a GM tube. Readings may be taken from the counter and corrected for background, allowing a graph of the decay to be plotted and hence the half-life to be measured.
published: 05 Dec 2009

Protactinium - Periodic Table of Videos
- Order: Reorder
- Duration: 7:06
- Uploaded Date: 14 Sep 2014
- views: 372696
- published: 14 Sep 2014
- views: 372696

What is PROTACTINIUM?
- Order: Reorder
- Duration: 2:54
- Uploaded Date: 04 Nov 2018
- views: 501
- published: 04 Nov 2018
- views: 501

Protactinium half life experiment
- Order: Reorder
- Duration: 7:50
- Uploaded Date: 18 Oct 2017
- views: 6863
- published: 18 Oct 2017
- views: 6863

Protactinium - Video Learning - WizScience.com
- Order: Reorder
- Duration: 2:59
- Uploaded Date: 10 Sep 2015
- views: 1452
- published: 10 Sep 2015
- views: 1452

Come Around
- Order: Reorder
- Duration: 4:19
- Uploaded Date: 29 Sep 2020
- views: 5138
- published: 29 Sep 2020
- views: 5138

Incredibly rare and radioactive elements ☢
- Order: Reorder
- Duration: 10:58
- Uploaded Date: 11 Apr 2019
- views: 454698
- published: 11 Apr 2019
- views: 454698

The Half-life of Protactinium
- Order: Reorder
- Duration: 5:41
- Uploaded Date: 05 Dec 2009
- views: 18328
- published: 05 Dec 2009
- views: 18328



Protactinium - Periodic Table of Videos
- Report rights infringement
- published: 14 Sep 2014
- views: 372696

What is PROTACTINIUM?
- Report rights infringement
- published: 04 Nov 2018
- views: 501

Protactinium half life experiment
- Report rights infringement
- published: 18 Oct 2017
- views: 6863

Protactinium - Video Learning - WizScience.com
- Report rights infringement
- published: 10 Sep 2015
- views: 1452

Come Around
- Report rights infringement
- published: 29 Sep 2020
- views: 5138

Incredibly rare and radioactive elements ☢
- Report rights infringement
- published: 11 Apr 2019
- views: 454698

The Half-life of Protactinium
- Report rights infringement
- published: 05 Dec 2009
- views: 18328

Protactinium
Protactinium is a chemical element with symbol Pa and atomic number 91. It is a dense, silvery-gray metal which readily reacts with oxygen, water vapor and inorganic acids. It forms various chemical compounds where protactinium is usually present in the oxidation state +5, but can also assume +4 and even +2 or +3 states. The average concentrations of protactinium in the Earth's crust is typically on the order of a few parts per trillion, but may reach up to a few parts per million in some uraninite ore deposits. Because of its scarcity, high radioactivity and high toxicity, there are currently no uses for protactinium outside of scientific research, and for this purpose, protactinium is mostly extracted from spent nuclear fuel.
Protactinium was first identified in 1913 by Kasimir Fajans and Oswald Helmuth Göhring and named brevium because of the short half-life of the specific isotope studied, namely protactinium-234. A more stable isotope (231Pa) of protactinium was discovered in 1917/18 by Otto Hahn and Lise Meitner, and they chose the name proto-actinium, but then the IUPAC named it finally protactinium in 1949 and confirmed Hahn and Meitner as discoverers. The new name meant "parent of actinium" and reflected the fact that actinium is a product of radioactive decay of protactinium. It is noted that John Arnold Cranston (working with Frederick Soddy) is also credited with discovering the stable isotope in 1915 but delayed his announcement due to being called up for service in the First World War.
