- published: 05 Mar 2015
- views: 150
-
remove the playlistUs Fda
-
remove the playlistFood And Drug Administration Travel Guides
-
remove the playlistLatest Videos
-
remove the playlistLongest Videos
- remove the playlistUs Fda
- remove the playlistFood And Drug Administration Travel Guides
- remove the playlistLatest Videos
- remove the playlistLongest Videos
- published: 20 Jul 2015
- views: 35249
- published: 30 May 2013
- views: 20067
- published: 18 Mar 2016
- views: 136
- published: 01 Aug 2016
- views: 177393
- published: 16 Mar 2017
- views: 11
- published: 20 Jul 2015
- views: 68568
- published: 22 Mar 2017
- views: 5816
- published: 11 Dec 2013
- views: 11998

Food and Drug Administration
The Food and Drug Administration (FDA or USFDA) is a federal agency of the United States Department of Health and Human Services, one of the United States federal executive departments. The FDA is responsible for protecting and promoting public health through the regulation and supervision of food safety, tobacco products, dietary supplements, prescription and over-the-counter pharmaceutical drugs (medications), vaccines, biopharmaceuticals, blood transfusions, medical devices, electromagnetic radiation emitting devices (ERED), cosmetics, animal foods & feed and veterinary products.
The FDA was empowered by the United States Congress to enforce the Federal Food, Drug, and Cosmetic Act, which serves as the primary focus for the Agency; the FDA also enforces other laws, notably Section 361 of the Public Health Service Act and associated regulations, many of which are not directly related to food or drugs. These include regulating lasers, cellular phones, condoms and control of disease on products ranging from certain household pets to sperm donation for assisted reproduction.
This article is licensed under the Creative Commons Attribution-ShareAlike 3.0 Unported License, which means that you can copy and modify it as long as the entire work (including additions) remains under this license.

U.S. Agent
U.S. Agent is a fictional comic book superhero appearing in books published by Marvel Comics, usually those starring Captain America and the Avengers. He was created by Mark Gruenwald and Paul Neary and first appeared in Captain America #323 (November 1986) as Super-Patriot. He was later redesigned as a new incarnation of Captain America and, just a few years later, as U.S.Agent. In 2012, U.S. Agent was ranked 29th in IGN's list of "The Top 50 Avengers".
Publication history
The character John Walker was first introduced as the supervillain Super-Patriot in Captain America #323. Mark Gruenwald created Walker to counter the general message in Captain America of patriotism being invariably good, describing him as someone
After a return appearance in Captain America #327, Gruenwald reintroduced him as the new Captain America in issue #333. Though Gruenwald said he would not have done this if it had not been a logical development from the preceding storylines, he also openly acknowledged that the motivating reason for replacing Steve Rogers as Captain America was to boost sales:
This article is licensed under the Creative Commons Attribution-ShareAlike 3.0 Unported License, which means that you can copy and modify it as long as the entire work (including additions) remains under this license.

United States
Coordinates: 40°N 100°W / 40°N 100°W / 40; -100
The United States of America (USA), commonly referred to as the United States (U.S.) or America, is a federal republic composed of 50 states, a federal district, five major territories and various possessions. The 48 contiguous states and Washington, D.C., are in central North America between Canada and Mexico. The state of Alaska is in the northwestern part of North America and the state of Hawaii is an archipelago in the mid-Pacific. The territories are scattered about the Pacific Ocean and the Caribbean Sea. At 3.8 million square miles (9.842 million km2) and with over 320 million people, the country is the world's third or fourth-largest by total area and the third most populous. It is one of the world's most ethnically diverse and multicultural nations, the product of large-scale immigration from many countries. The geography and climate of the United States are also extremely diverse, and the country is home to a wide variety of wildlife.
This article is licensed under the Creative Commons Attribution-ShareAlike 3.0 Unported License, which means that you can copy and modify it as long as the entire work (including additions) remains under this license.

FDA Food Safety Modernization Act
The FDA Food Safety Modernization Act of 2010 (FSMA) was signed into law by President Barack Obama on January 4, 2011. It aims to ensure the U.S. food supply is safe by shifting the focus of federal regulators from responding to contamination to preventing it. The FSMA has given the Food and Drug Administration (FDA) new authorities to regulate the way foods are grown, harvested and processed. The law grants FDA a number of new powers, including mandatory recall authority, which the agency has sought for many years. The FSMA requires FDA to undertake more than a dozen rulemakings and issue at least 10 guidance documents, as well as a host of reports, plans, strategies, standards, notices, and other tasks.
The law was prompted after many reported incidents of food-borne illnesses during the first decade of the 2000s. Tainted food has cost the food industry billions of dollars in recalls, lost sales and legal expenses.
This bill is similar to the Food Safety Enhancement Act which passed the House in 2009. It is considered the first major piece of federal legislation addressing food safety since 1938. It is also the first piece of legislation to address intentional adulteration and Food Defense.
This article is licensed under the Creative Commons Attribution-ShareAlike 3.0 Unported License, which means that you can copy and modify it as long as the entire work (including additions) remains under this license.

Food safety
Food safety is a scientific discipline describing handling, preparation, and storage of food in ways that prevent foodborne illness. This includes a number of routines that should be followed to avoid potentially severe health hazards. In this way Food Safety often overlaps with Food Defense to prevent harm to consumers. The tracks within this line of thought are safety between industry and the market and then between the market and the consumer. In considering industry to market practices, food safety considerations include the origins of food including the practices relating to food labeling, food hygiene, food additives and pesticide residues, as well as policies on biotechnology and food and guidelines for the management of governmental import and export inspection and certification systems for foods. In considering market to consumer practices, the usual thought is that food ought to be safe in the market and the concern is safe delivery and preparation of the food for the consumer.
This article is licensed under the Creative Commons Attribution-ShareAlike 3.0 Unported License, which means that you can copy and modify it as long as the entire work (including additions) remains under this license.
- Loading...

-
 1:59
1:59Introduction to the US FDA
Introduction to the US FDAIntroduction to the US FDA
Course Description: This course examines the range of the US FDA’s responsibilities and provides a chronological history of significant events regarding the US FDA and medical devices. It lays the foundation by exploring the regulations found in 21 Code of Federal Regulations (CFR) and the various pathways of marketing medical devices in the US. Learn more at: http://www.wmdo.org/course-detail.aspx?id=52 -
 7:38
7:38How Does the FDA Approve a Drug?
How Does the FDA Approve a Drug?How Does the FDA Approve a Drug?
You can directly support Healthcare Triage on Patreon: http://vid.io/xqXr If you can afford to pay a little every month, it really helps us to continue producing great content. Have you ever taken an over the counter medication for heartburn? How about an antibiotic for an ear infection? At some point pretty much all of us have visited a pharmacy to pick up a drug, but likely didn't consider where these drugs come from or how they are made. Whether you're talking about something for seasonal allergies or your grandparent's arthritis medication, the act of bringing a drug to market is long and complex. I'm not an expert, but HCT intern Rachel Hoffman is, and with her help, that's the topic of this week's Healthcare Triage. For those of you who want to read more, go here: http://theincidentaleconomist.com/wordpress/?p=63822 John Green -- Executive Producer Stan Muller -- Director, Producer Aaron Carroll -- Writer Mark Olsen -- Graphics http://www.twitter.com/aaronecarroll http://www.twitter.com/crashcoursestan http://www.twitter.com/johngreen http://www.twitter.com/olsenvideo And the housekeeping: 1) You can support Healthcare Triage on Patreon: http://vid.io/xqXr Every little bit helps make the show better! 2) Check out our Facebook page: http://goo.gl/LnOq5z 3) We still have merchandise available at http://www.hctmerch.com -
 23:50
23:50FDA Inspection Do and Don't List
FDA Inspection Do and Don't ListFDA Inspection Do and Don't List
If you have a FDA Inspection scheduled, you should prepare your staff. This video will show you what to do and what not to do during your FDA Inspection. If you need additional help or guidance, contact Compliance Insight. You can learn more about FDA Inspections here http://compliance-insight.com/fda-483-warning-letters/fda-483-inspection/ -
 10:40
10:40Indian pharma under dark clouds of US FDA
Indian pharma under dark clouds of US FDAIndian pharma under dark clouds of US FDA
DG Shah, secretary general, Indian Pharmaceutical Alliance (IPA), talks to NDTV about the spike in observations and warning letters from US FDA to Indian pharma industry. He says that the number of facilities have gone up and hence an increase. The industry has identified the key issues and is talking several measures to resolve this problem. Now he believes it is just a matter of time. He also spoke about the banning of several drugs by the government and said that due process was not followed and there was no transparency. Watch more videos: http://profit.ndtv.com/videos?yt Download the NDTV news app: https://play.google.com/store/apps/details?id=com.july.ndtv&referrer;=utm_source%3Dyoutubecards%26utm_medium%3Dcpc%26utm_campaign%3Dyoutube -
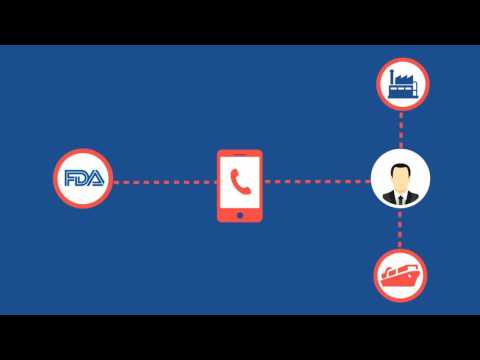 3:05
3:05U.S. FDA Food Facility Registration Renewal
U.S. FDA Food Facility Registration RenewalU.S. FDA Food Facility Registration Renewal
Under the Food Safety Modernization Act (FSMA), the U.S. Food and Drug Administration (FDA) requires all food facilities registered with FDA to renew their registrations between October 1 and December 31 of every even-numbered year. Transcript: On January 4th, 2011, the United States President signed into law the Food Safety Modernization Act. This law requires all food facilities registered with the U.S. Food and Drug Administration to renew their registrations between October 1st and December 31st of each even-numbered year. Failure to renew a facility registration, update required elements, or cancel a registration in accordance with this regulation, is a prohibited act. As part of a facility’s registration renewal, food facilities located outside the United States must provide contact details for a U.S. Agent for FDA communications. The function and responsibility of a U.S. Agent for FDA communications are different from those of a commercial agent, such as an importer or broker. A U.S. Agent for FDA purposes facilitates communication between FDA and the foreign food facility. The U.S. Agent must be available for FDA to contact 24 hours a day, 7 days a week. FDA will contact the U.S. Agent to schedule inspections of the foreign facility, or even to ask questions about a specific shipment of food to the U.S. It is important to designate a U.S. Agent with expertise in FDA regulations, since the U.S. Agent may speak on behalf of the registered foreign facility. By renewing your FDA registration, you also grant FDA the right to inspect your facility. Not only will the inspector seek to verify that your registration information is accurate, but he will also verify other FDA requirements, such as Good Manufacturing Practices, Labeling Compliance, and compliance with the Food Safety Modernization Act. Under the Food Safety Modernization Act, FDA has been ordered to increase the number of foreign facility inspections it conducts. If you receive an FDA Notice of Inspection, Registrar Corp can help you prepare. As a part of our U.S. Agent service for foreign facilities, Registrar Corp will dispatch a food safety expert trained in FDA inspections to your facility. This assistance is included at no additional charge, other than travel and lodging expenses, when FDA schedules an inspection. While renewing a registration may sound simple, a number of mistakes are frequently made. Mistakes in registrations can have serious consequences for your business, including detentions of your products or suspension of your registration. Registrar Corp’s Regulatory Specialists can help you avoid these consequences by renewing your FDA registration properly. If you would like more information about the registration renewal of your food facility, retaining Registrar Corp as your U.S. Agent, or answers to other questions about FDA regulations, please feel free to contact Registrar Corp. Simply phone our U.S. office at 757-224-0177 or go to our website at www.registrarcorp.com. -
 1:28:01
1:28:01New U.S. FDA Food Labeling Rules
New U.S. FDA Food Labeling RulesNew U.S. FDA Food Labeling Rules
In order to help food companies properly label their products for U.S. distribution, Registrar Corp compiled a list of some U.S. Food and Drug Administration (FDA) food and beverage labeling regulations. Join Registrar Corp to learn more about the requirements for compliance with the new FDA food labeling rules. -
 3:29
3:29How Does The FDA Approve New Drugs?
How Does The FDA Approve New Drugs?How Does The FDA Approve New Drugs?
Is China Fueling The Drug War? http://testu.be/1GzTYHN Subscribe! http://bitly.com/1iLOHml The Food and Drug Administration is one of the world's strictest regulatory agencies. So, how do drugs get approved for use in the U.S.? Learn More: Drug Safety/Drug Approval Process http://www.fda.gov/NewsEvents/Testimony/ucm161673.htm "Modern drugs provide unmistakable and significant health benefits." House overwhelmingly passes bill to speed FDA drug approvals http://www.washingtonpost.com/blogs/wonkblog/wp/2015/07/10/house-overwhelmingly-passes-bill-to-speed-fda-drug-approvals/ "A bipartisan bill that would make significant changes to the process for developing new drugs and medical devices overwhelmingly passed the House in a 344-77 vote Friday morning." Novel New Drugs 2013 Summary http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DrugInnovation/UCM381803.pdf "In Calendar Year 2013, FDA's Center for Drug Evaluation and Research (CDER) approved 27 novel new medicines, known as new molecular entities (NMEs)." As drug making goes global, oversight found lacking http://www.usatoday.com/story/news/2012/10/21/global-drug-manufacturing-oversight/1646487/ "The medicines you took this morning could have come from anywhere, and everywhere, in the world." Subscribe to TestTube Daily! http://bitly.com/1iLOHml _________________________ TestTube News is committed to answering the smart, inquisitive questions we have about life, society, politics and anything else happening in the news. It's a place where curiosity rules and together we'll get a clearer understanding of this crazy world we live in. Watch more TestTube: http://testtube.com/testtubenews Subscribe now! http://www.youtube.com/subscription_center?add_user=testtubenetwork TestTube on Twitter https://twitter.com/TestTube Trace Dominguez on Twitter https://twitter.com/TraceDominguez TestTube on Facebook https://facebook.com/testtubenetwork TestTube on Google+ http://gplus.to/TestTube Download the New TestTube iOS app! http://testu.be/1ndmmMq Special thanks to Julia Wilde for hosting TestTube! Check Julia on Twitter: https://twitter.com/Julia_SCI -
 7:34
7:34Brian G Nadel, Former Investigator, USFDA & Consultant || Pharma Regulators' Day || PhilipCapital
Brian G Nadel, Former Investigator, USFDA & Consultant || Pharma Regulators' Day || PhilipCapitalBrian G Nadel, Former Investigator, USFDA & Consultant || Pharma Regulators' Day || PhilipCapital
Nikitsha Chopra, PING Network in conversation with Brian G Nadel, Former Investigator, USFDA & Consultant talking Pharma Regulators' Day 2017. To start Investing with financial expert Phillip Capital at http://www.phillipcapital.in/Open-An-Account Subscribe to our channel for more updates and interesting videos ! -
 3:32
3:32Triclosan found in 75% of us, FDA knows it's dangerous
Triclosan found in 75% of us, FDA knows it's dangerousTriclosan found in 75% of us, FDA knows it's dangerous
The FDA first proposed that the chemical Triclosan be removed from consumer products in 1978, in reaction to several studies that showed it to be an endocrine disruptor, with potential dangerous effects on development and reproduction. They took no further action. In 1997, they approved Colgate Total for market, even though it contained Triclosan. The NRDC filed to see the paperwork for this approval under FOIA. The FDA has failed to comply, and now the NRDC is suing the FDA. Let's just hope it doesn't take another 35 years for the FDA to start regulating the potentially dangerous chemical. The Resident (aka Lori Harfenist) discusses. Follow The Resident at http://www.twitter.com/TheResident Find RT America in your area: http://rt.com/where-to-watch/ Or watch us online: http://rt.com/on-air/rt-america-air/ Like us on Facebook http://www.facebook.com/RTAmerica Follow us on Twitter http://twitter.com/RT_America -
 3:57
3:57US FDA Regulaciones de Etiquetado para Alimentos y Bebidas Español
US FDA Regulaciones de Etiquetado para Alimentos y Bebidas EspañolUS FDA Regulaciones de Etiquetado para Alimentos y Bebidas Español
-

Introduction to the US FDA
Course Description: This course examines the range of the US FDA’s responsibilities and provides a chronological history of significant events regarding the US FDA and medical devices. It lays the foundation by exploring the regulations found in 21 Code of Federal Regulations (CFR) and the various pathways of marketing medical devices in the US. Learn more at: http://www.wmdo.org/course-detail.aspx?id=52
published: 05 Mar 2015 -

How Does the FDA Approve a Drug?
You can directly support Healthcare Triage on Patreon: http://vid.io/xqXr If you can afford to pay a little every month, it really helps us to continue producing great content. Have you ever taken an over the counter medication for heartburn? How about an antibiotic for an ear infection? At some point pretty much all of us have visited a pharmacy to pick up a drug, but likely didn't consider where these drugs come from or how they are made. Whether you're talking about something for seasonal allergies or your grandparent's arthritis medication, the act of bringing a drug to market is long and complex. I'm not an expert, but HCT intern Rachel Hoffman is, and with her help, that's the topic of this week's Healthcare Triage. For those of you who want to read more, go here: http://theinci...
published: 20 Jul 2015 -

FDA Inspection Do and Don't List
If you have a FDA Inspection scheduled, you should prepare your staff. This video will show you what to do and what not to do during your FDA Inspection. If you need additional help or guidance, contact Compliance Insight. You can learn more about FDA Inspections here http://compliance-insight.com/fda-483-warning-letters/fda-483-inspection/
published: 30 May 2013 -

Indian pharma under dark clouds of US FDA
DG Shah, secretary general, Indian Pharmaceutical Alliance (IPA), talks to NDTV about the spike in observations and warning letters from US FDA to Indian pharma industry. He says that the number of facilities have gone up and hence an increase. The industry has identified the key issues and is talking several measures to resolve this problem. Now he believes it is just a matter of time. He also spoke about the banning of several drugs by the government and said that due process was not followed and there was no transparency. Watch more videos: http://profit.ndtv.com/videos?yt Download the NDTV news app: https://play.google.com/store/apps/details?id=com.july.ndtv&referrer;=utm_source%3Dyoutubecards%26utm_medium%3Dcpc%26utm_campaign%3Dyoutube
published: 18 Mar 2016 -

U.S. FDA Food Facility Registration Renewal
Under the Food Safety Modernization Act (FSMA), the U.S. Food and Drug Administration (FDA) requires all food facilities registered with FDA to renew their registrations between October 1 and December 31 of every even-numbered year. Transcript: On January 4th, 2011, the United States President signed into law the Food Safety Modernization Act. This law requires all food facilities registered with the U.S. Food and Drug Administration to renew their registrations between October 1st and December 31st of each even-numbered year. Failure to renew a facility registration, update required elements, or cancel a registration in accordance with this regulation, is a prohibited act. As part of a facility’s registration renewal, food facilities located outside the United States must provid...
published: 01 Aug 2016 -

New U.S. FDA Food Labeling Rules
In order to help food companies properly label their products for U.S. distribution, Registrar Corp compiled a list of some U.S. Food and Drug Administration (FDA) food and beverage labeling regulations. Join Registrar Corp to learn more about the requirements for compliance with the new FDA food labeling rules.
published: 16 Mar 2017 -

How Does The FDA Approve New Drugs?
Is China Fueling The Drug War? http://testu.be/1GzTYHN Subscribe! http://bitly.com/1iLOHml The Food and Drug Administration is one of the world's strictest regulatory agencies. So, how do drugs get approved for use in the U.S.? Learn More: Drug Safety/Drug Approval Process http://www.fda.gov/NewsEvents/Testimony/ucm161673.htm "Modern drugs provide unmistakable and significant health benefits." House overwhelmingly passes bill to speed FDA drug approvals http://www.washingtonpost.com/blogs/wonkblog/wp/2015/07/10/house-overwhelmingly-passes-bill-to-speed-fda-drug-approvals/ "A bipartisan bill that would make significant changes to the process for developing new drugs and medical devices overwhelmingly passed the House in a 344-77 vote Friday morning." Novel New Drugs 2013 Summary htt...
published: 20 Jul 2015 -

Brian G Nadel, Former Investigator, USFDA & Consultant || Pharma Regulators' Day || PhilipCapital
Nikitsha Chopra, PING Network in conversation with Brian G Nadel, Former Investigator, USFDA & Consultant talking Pharma Regulators' Day 2017. To start Investing with financial expert Phillip Capital at http://www.phillipcapital.in/Open-An-Account Subscribe to our channel for more updates and interesting videos !
published: 22 Mar 2017 -

Triclosan found in 75% of us, FDA knows it's dangerous
The FDA first proposed that the chemical Triclosan be removed from consumer products in 1978, in reaction to several studies that showed it to be an endocrine disruptor, with potential dangerous effects on development and reproduction. They took no further action. In 1997, they approved Colgate Total for market, even though it contained Triclosan. The NRDC filed to see the paperwork for this approval under FOIA. The FDA has failed to comply, and now the NRDC is suing the FDA. Let's just hope it doesn't take another 35 years for the FDA to start regulating the potentially dangerous chemical. The Resident (aka Lori Harfenist) discusses. Follow The Resident at http://www.twitter.com/TheResident Find RT America in your area: http://rt.com/where-to-watch/ Or watch us online: http://rt.com/on-a...
published: 11 Dec 2013 -

US FDA Regulaciones de Etiquetado para Alimentos y Bebidas Español
published: 05 Jan 2015
Introduction to the US FDA
- Order: Reorder
- Duration: 1:59
- Updated: 05 Mar 2015
- views: 150
- published: 05 Mar 2015
- views: 150
How Does the FDA Approve a Drug?
- Order: Reorder
- Duration: 7:38
- Updated: 20 Jul 2015
- views: 35249
- published: 20 Jul 2015
- views: 35249
FDA Inspection Do and Don't List
- Order: Reorder
- Duration: 23:50
- Updated: 30 May 2013
- views: 20067
- published: 30 May 2013
- views: 20067
Indian pharma under dark clouds of US FDA
- Order: Reorder
- Duration: 10:40
- Updated: 18 Mar 2016
- views: 136
- published: 18 Mar 2016
- views: 136
U.S. FDA Food Facility Registration Renewal
- Order: Reorder
- Duration: 3:05
- Updated: 01 Aug 2016
- views: 177393
- published: 01 Aug 2016
- views: 177393
New U.S. FDA Food Labeling Rules
- Order: Reorder
- Duration: 1:28:01
- Updated: 16 Mar 2017
- views: 11
- published: 16 Mar 2017
- views: 11
How Does The FDA Approve New Drugs?
- Order: Reorder
- Duration: 3:29
- Updated: 20 Jul 2015
- views: 68568
- published: 20 Jul 2015
- views: 68568
Brian G Nadel, Former Investigator, USFDA & Consultant || Pharma Regulators' Day || PhilipCapital
- Order: Reorder
- Duration: 7:34
- Updated: 22 Mar 2017
- views: 5816
- published: 22 Mar 2017
- views: 5816
Triclosan found in 75% of us, FDA knows it's dangerous
- Order: Reorder
- Duration: 3:32
- Updated: 11 Dec 2013
- views: 11998
- published: 11 Dec 2013
- views: 11998
US FDA Regulaciones de Etiquetado para Alimentos y Bebidas Español
- Order: Reorder
- Duration: 3:57
- Updated: 05 Jan 2015
- views: 40
- published: 05 Jan 2015
- views: 40
-

Have Questions About Traveling with Medication? The FDA Has Answers
www.rxwiki.com Traveling with medication can be tricky — especially when it comes to bringing any into the US from a different country. To clear up some questions regarding the legality of various situations, the US Food and Drug Administration (FDA) has released a few tips for people seeking to bring medication into the country.
published: 08 Feb 2016 -

12 Days in Europe in a Carry-On?! // Packing Challenge!
Guess what?! I'm doing Vlogmas! Check it out starting Tuesday, December 13th, on EllesGlitterGossip. vitafusion™ disclaimer: These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure or prevent any disease. If you like packing videos, make sure to watch this playlist!!! https://www.youtube.com/playlist?list=PLsobo-3H369FUDTGfcNyAZXMnAMMu_fww Wanna know where I've been? Check out my planner channel! http://www.youtube.com/glamplanner Snapchat: @missellefowler Tweet me, I love twitter: http://www.twitter.com/ellefowler Or tag me on Instagram: http://instagram.com/missellefowler _________________________________________________ Subscribe to my second channel for random vlogs, book reviews, healthy tips, and ador...
published: 13 Dec 2016 -

Secrets of the FDA: The Business and Politics Behind the Food We Eat (2005)
The Food and Drug Administration (FDA or USFDA) is a federal agency of the United States Department of Health and Human Services, one of the United States federal executive departments. About the book: https://www.amazon.com/gp/product/0471610917/ref=as_li_tl?ie=UTF8&camp;=1789&creative;=9325&creativeASIN;=0471610917&linkCode;=as2&tag;=tra0c7-20&linkId;=c1ccc5804f9252857f9a27e635dcd1ae The FDA is responsible for protecting and promoting public health through the control and supervision of food safety, tobacco products, dietary supplements, prescription and over-the-counter pharmaceutical drugs (medications), vaccines, biopharmaceuticals, blood transfusions, medical devices, electromagnetic radiation emitting devices (ERED), cosmetics, animal foods & feed[5] and veterinary products. The FDA was...
published: 26 Jan 2017 -

Top 10 Travel Essentials | For the Airplane!
My essentials and necessities I bring with me on the airplane whenever I travel! COLLABORATION ▼◆◀︎ Thank you Collective Bias, Inc and Emergen-C for working with me on this video! #EmergenCRecipes #CollectiveBias FIND OUT MORE ABOUT EMERGEN-C ▼◆◀︎ ▸ PURCHASE ‣ http://cbi.as/440he ▸ BRAND INFO ‣ http://cbi.as/440ie These statements have not been evaluated by the Food and Drug Administration. These products are not intended to diagnose, treat, cure or prevent any disease. SUBSCRIBE ▸ http://www.youtube.com/user/lovehealthfitness?sub_confirmation=1 ADD ME ▼◆◀︎ ▸ SNAPCHAT ‣ lovehealthok ▸ INSTAGRAM ‣ http://instagram.com/lovehealthok ▸ TWITTER ‣ http://twitter.com/lovehealthok ▸ FACEBOOK ‣ http://facebook.com/lovehealthok ▸ PINTEREST ‣ http://pinterest.com/lovehealthok MORE VIDEO...
published: 20 Dec 2016 -

Basic Guide to Exporting: Understanding of Free Sale Export Compliance
In this webinar, you will learn that the Free Sale and Export Health Certificates are two common export documents required when exporting live animals, food, drugs, and sometimes medical or veterinary equipment. Issued by the FDA, USDA, or State Ag offices, this webinar will teach you about where to turn within each of these departments to help you certify your products for export. For more: http://export.gov/webinars/eg_main_072745.asp July 16, 2013
published: 19 Jun 2014 -

Improving food safety at Jordan's hotels and restaurants
The USAID tourism project worked closely with the Jordan Food and Drug Administration and other stakeholders to develop national food safety guidelines and implement these at the country's hotels and restaurants.
published: 20 Jan 2015 -

-

Nutrition Labels are Changing!
The FDA has approved a new nutrition facts panel! So what's changing and what does it mean for us? Subscribe for more videos: http://bit.ly/1OExX5z The new nutrition facts panel has been in the works for a couple years now, but the the FDA has finally approved the updated design! There have been a few changes made to the overall appearance of the panel and some changes to what information is included. They've also made some important updates to serving sizes and the way they're used. This is big stuff! Food companies will be required to use the new nutrition facts panel starting June 26, 2018. Pictures and more info on the updated nutrition facts panel: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm502182.htm FDA press release: http://www.fda.gov/NewsEvents/Newsroom/Pres...
published: 21 May 2016 -

Final Rule for Sanitary Transportation
The U.S. Food & Drug Administration (FDA) hosted a webinar on April 25, 2016, soon after the release of the Food Safety Modernization Act (FSMA) Final Rule on Sanitary Transportation of Human and Animal Food. This final rule is part of FDA’s implementation of the 2005 Sanitary Food Transportation Act. It builds on current food transportation best practices and is focused on ensuring that the individuals transporting food at the greatest risk for contamination during transportation follow appropriate sanitary transportation practices.
published: 12 May 2016 -

Budget travel greece
Budget travel greece - Financial and Shopping Tips When Traveling Overseas Glazed Ceramics Beware of purchasing glazed ceramic ware abroad. It is possible to suffer lead poisoning, if you consume food or beverages that are stored or served in improperly glazed ceramics. Unless the ceramics are made by a firm with an international reputation, there is no immediate way to be certain that a particular item is safe. The U.S. Food and Drug Administration recommends that ceramic tableware purchased abroad be tested for lead release by a commercial laboratory on your return or be used for decorative purposes only. Related Videos: Budget travel greece http://youtu.be/LGgVf6yuz9s http://youtu.be/TYnLOeEV0Bk Related Blogs: Other Videos: Budget travel honolulu : 00:00:05 Budget travel hone...
published: 11 Oct 2014
Have Questions About Traveling with Medication? The FDA Has Answers
- Order: Reorder
- Duration: 1:47
- Updated: 08 Feb 2016
- views: 0
- published: 08 Feb 2016
- views: 0
12 Days in Europe in a Carry-On?! // Packing Challenge!
- Order: Reorder
- Duration: 13:01
- Updated: 13 Dec 2016
- views: 136312
- published: 13 Dec 2016
- views: 136312
Secrets of the FDA: The Business and Politics Behind the Food We Eat (2005)
- Order: Reorder
- Duration: 1:00:05
- Updated: 26 Jan 2017
- views: 364
- published: 26 Jan 2017
- views: 364
Top 10 Travel Essentials | For the Airplane!
- Order: Reorder
- Duration: 3:14
- Updated: 20 Dec 2016
- views: 3754
- published: 20 Dec 2016
- views: 3754
Basic Guide to Exporting: Understanding of Free Sale Export Compliance
- Order: Reorder
- Duration: 1:04:51
- Updated: 19 Jun 2014
- views: 1539
- published: 19 Jun 2014
- views: 1539
Improving food safety at Jordan's hotels and restaurants
- Order: Reorder
- Duration: 4:12
- Updated: 20 Jan 2015
- views: 520
- published: 20 Jan 2015
- views: 520
FDA CTP Webinar 6.26.2014
- Order: Reorder
- Duration: 1:19:38
- Updated: 24 Nov 2014
- views: 814
Nutrition Labels are Changing!
- Order: Reorder
- Duration: 7:24
- Updated: 21 May 2016
- views: 198
- published: 21 May 2016
- views: 198
Final Rule for Sanitary Transportation
- Order: Reorder
- Duration: 1:01:04
- Updated: 12 May 2016
- views: 1960
- published: 12 May 2016
- views: 1960
Budget travel greece
- Order: Reorder
- Duration: 0:49
- Updated: 11 Oct 2014
- views: 9
- published: 11 Oct 2014
- views: 9
-

Introduction to the US FDA
Course Description: This course examines the range of the US FDA’s responsibilities and provides a chronological history of significant events regarding the US FDA and medical devices. It lays the foundation by exploring the regulations found in 21 Code of Federal Regulations (CFR) and the various pathways of marketing medical devices in the US. Learn more at: http://www.wmdo.org/course-detail.aspx?id=52
published: 05 Mar 2015 -

How Does the FDA Approve a Drug?
You can directly support Healthcare Triage on Patreon: http://vid.io/xqXr If you can afford to pay a little every month, it really helps us to continue producing great content. Have you ever taken an over the counter medication for heartburn? How about an antibiotic for an ear infection? At some point pretty much all of us have visited a pharmacy to pick up a drug, but likely didn't consider where these drugs come from or how they are made. Whether you're talking about something for seasonal allergies or your grandparent's arthritis medication, the act of bringing a drug to market is long and complex. I'm not an expert, but HCT intern Rachel Hoffman is, and with her help, that's the topic of this week's Healthcare Triage. For those of you who want to read more, go here: http://theinci...
published: 20 Jul 2015 -

FDA Inspection Do and Don't List
If you have a FDA Inspection scheduled, you should prepare your staff. This video will show you what to do and what not to do during your FDA Inspection. If you need additional help or guidance, contact Compliance Insight. You can learn more about FDA Inspections here http://compliance-insight.com/fda-483-warning-letters/fda-483-inspection/
published: 30 May 2013 -

Indian pharma under dark clouds of US FDA
DG Shah, secretary general, Indian Pharmaceutical Alliance (IPA), talks to NDTV about the spike in observations and warning letters from US FDA to Indian pharma industry. He says that the number of facilities have gone up and hence an increase. The industry has identified the key issues and is talking several measures to resolve this problem. Now he believes it is just a matter of time. He also spoke about the banning of several drugs by the government and said that due process was not followed and there was no transparency. Watch more videos: http://profit.ndtv.com/videos?yt Download the NDTV news app: https://play.google.com/store/apps/details?id=com.july.ndtv&referrer;=utm_source%3Dyoutubecards%26utm_medium%3Dcpc%26utm_campaign%3Dyoutube
published: 18 Mar 2016 -

U.S. FDA Food Facility Registration Renewal
Under the Food Safety Modernization Act (FSMA), the U.S. Food and Drug Administration (FDA) requires all food facilities registered with FDA to renew their registrations between October 1 and December 31 of every even-numbered year. Transcript: On January 4th, 2011, the United States President signed into law the Food Safety Modernization Act. This law requires all food facilities registered with the U.S. Food and Drug Administration to renew their registrations between October 1st and December 31st of each even-numbered year. Failure to renew a facility registration, update required elements, or cancel a registration in accordance with this regulation, is a prohibited act. As part of a facility’s registration renewal, food facilities located outside the United States must provid...
published: 01 Aug 2016 -

New U.S. FDA Food Labeling Rules
In order to help food companies properly label their products for U.S. distribution, Registrar Corp compiled a list of some U.S. Food and Drug Administration (FDA) food and beverage labeling regulations. Join Registrar Corp to learn more about the requirements for compliance with the new FDA food labeling rules.
published: 16 Mar 2017 -

How Does The FDA Approve New Drugs?
Is China Fueling The Drug War? http://testu.be/1GzTYHN Subscribe! http://bitly.com/1iLOHml The Food and Drug Administration is one of the world's strictest regulatory agencies. So, how do drugs get approved for use in the U.S.? Learn More: Drug Safety/Drug Approval Process http://www.fda.gov/NewsEvents/Testimony/ucm161673.htm "Modern drugs provide unmistakable and significant health benefits." House overwhelmingly passes bill to speed FDA drug approvals http://www.washingtonpost.com/blogs/wonkblog/wp/2015/07/10/house-overwhelmingly-passes-bill-to-speed-fda-drug-approvals/ "A bipartisan bill that would make significant changes to the process for developing new drugs and medical devices overwhelmingly passed the House in a 344-77 vote Friday morning." Novel New Drugs 2013 Summary htt...
published: 20 Jul 2015 -

Brian G Nadel, Former Investigator, USFDA & Consultant || Pharma Regulators' Day || PhilipCapital
Nikitsha Chopra, PING Network in conversation with Brian G Nadel, Former Investigator, USFDA & Consultant talking Pharma Regulators' Day 2017. To start Investing with financial expert Phillip Capital at http://www.phillipcapital.in/Open-An-Account Subscribe to our channel for more updates and interesting videos !
published: 22 Mar 2017 -

Triclosan found in 75% of us, FDA knows it's dangerous
The FDA first proposed that the chemical Triclosan be removed from consumer products in 1978, in reaction to several studies that showed it to be an endocrine disruptor, with potential dangerous effects on development and reproduction. They took no further action. In 1997, they approved Colgate Total for market, even though it contained Triclosan. The NRDC filed to see the paperwork for this approval under FOIA. The FDA has failed to comply, and now the NRDC is suing the FDA. Let's just hope it doesn't take another 35 years for the FDA to start regulating the potentially dangerous chemical. The Resident (aka Lori Harfenist) discusses. Follow The Resident at http://www.twitter.com/TheResident Find RT America in your area: http://rt.com/where-to-watch/ Or watch us online: http://rt.com/on-a...
published: 11 Dec 2013 -

US FDA Regulaciones de Etiquetado para Alimentos y Bebidas Español
published: 05 Jan 2015
Introduction to the US FDA
- Order: Reorder
- Duration: 1:59
- Updated: 05 Mar 2015
- views: 150
- published: 05 Mar 2015
- views: 150
How Does the FDA Approve a Drug?
- Order: Reorder
- Duration: 7:38
- Updated: 20 Jul 2015
- views: 35249
- published: 20 Jul 2015
- views: 35249
FDA Inspection Do and Don't List
- Order: Reorder
- Duration: 23:50
- Updated: 30 May 2013
- views: 20067
- published: 30 May 2013
- views: 20067
Indian pharma under dark clouds of US FDA
- Order: Reorder
- Duration: 10:40
- Updated: 18 Mar 2016
- views: 136
- published: 18 Mar 2016
- views: 136
U.S. FDA Food Facility Registration Renewal
- Order: Reorder
- Duration: 3:05
- Updated: 01 Aug 2016
- views: 177393
- published: 01 Aug 2016
- views: 177393
New U.S. FDA Food Labeling Rules
- Order: Reorder
- Duration: 1:28:01
- Updated: 16 Mar 2017
- views: 11
- published: 16 Mar 2017
- views: 11
How Does The FDA Approve New Drugs?
- Order: Reorder
- Duration: 3:29
- Updated: 20 Jul 2015
- views: 68568
- published: 20 Jul 2015
- views: 68568
Brian G Nadel, Former Investigator, USFDA & Consultant || Pharma Regulators' Day || PhilipCapital
- Order: Reorder
- Duration: 7:34
- Updated: 22 Mar 2017
- views: 5816
- published: 22 Mar 2017
- views: 5816
Triclosan found in 75% of us, FDA knows it's dangerous
- Order: Reorder
- Duration: 3:32
- Updated: 11 Dec 2013
- views: 11998
- published: 11 Dec 2013
- views: 11998
US FDA Regulaciones de Etiquetado para Alimentos y Bebidas Español
- Order: Reorder
- Duration: 3:57
- Updated: 05 Jan 2015
- views: 40
- published: 05 Jan 2015
- views: 40
-

Mobile Medical Apps & the US FDA Regulatory Oversight Still a Thin Red Line 20140914
published: 07 Feb 2015 -

US FDA Dr. Robert Califf Opens MDIC 2015 Annual Public Forum
MDIC CEO Bill Murray opens the MDIC 2015 Annual Public Forum and introduces keynote speaker US FDA Deputy Commissioner Dr. Robert Califf
published: 04 Nov 2015 -

Band Baaja Bride show on NDTV Good Times (ft. Dr Chytra V Anand)
The show features Dr Chytra V Anand. Join our social pages: http://www.facebook.com/Kosmoderma https://twitter.com/drchytra https://twitter.com/kosmoderma http://blog.kosmoderma.com/ Kosmoderma Clinics are dedicated to medical excellence in the field of Cosmetic Dermatology with international accreditation and facilities. We offer scientifically proven treatments using US FDA & European CE approved technologies.
published: 21 Mar 2014 -

-

2. Основы концепции биоэквивалентности
Видео, состоящее из двух частей, посвящено основам концепции биоэквивалентности как способа экстраполяции данных по безопасности и эффективности одного лекарственного препарата на другой на основании данных о сопоставимой биодоступности (часть 2/2). Использованная литература Oral drug absorption : prediction and assessment. Jennifer B. Dressman, Christos Reppas (Eds). — 2nd ed. Informa Healthcare USA, Inc., New York. 2010 Biopharmaceutics Applications in Drug Development. Rajesh Krishna, Lawrence Yu (Eds.). Springer Science+Business Media, LLC. 2008 Hauschke, Dieter. Bioequivalence studies in drug development : methods and applications / Dieter Hauschke, Volker Steinijans, Iris Pigeot. p. ; cm. John Wiley & Sons Ltd. 2007 Shein-Chung Chow, Jen-pei Liu. Design and Analysis of Bioavailabili...
published: 12 Jul 2016 -

Limecrime Velvetine Review with Check Ins (Pink Velvet) & Controversy
Hi guys thanks for tuning in! Please subscribe if you like it! Details Below ↘️ Click show more ↘️↘️↘️ My views on the controversy starts at 10:22 Removal is at 34:19 ♡ Where you can Stalk me ♡ Instagram: SF_Stef http://instagram.com/SF_stef Facebook: Stephanie Nicole https://www.facebook.com/pages/Stephanie-Nicole/430018237078644 Twitter: @StefNicole https://twitter.com/stefnicole Snapchat: Sf.stef ***Check out my current liquid lipstick review series on the following brands: **ABH Liquid Lips http://bit.ly/1IWmLNi **Check out my second chance review of ABH Liquid Lips - http://bit.ly/1jmdELv **Dose of Colors Matte Lipstick http://bit.ly/1H1xup7 **Kat Von D Everlasting Lipstick http://bit.ly/1PxezBY **Stila Stay All Day Lipstick - http://bit.ly/1IFMf1S **Sephora Cream Lip...
published: 30 Jun 2015 -

-

Band Baaja Bride show on NDTV Good Times (ft. Dr Chytra V Anand)
The show features Dr Chytra V Anand. Join our social pages: http://www.facebook.com/Kosmoderma https://twitter.com/drchytra https://twitter.com/kosmoderma http://blog.kosmoderma.com/ Kosmoderma Clinics are dedicated to medical excellence in the field of Cosmetic Dermatology with international accreditations and facilities. We offer scientifically proven treatments using US FDA & European CE approved technologies.
published: 15 Apr 2013 -

PQE - QUALITY DRIVEN DATA INTEGRITY APPROACH
The following presentation was conducted by leading data integrity expert and PQE CEO, Gilda D'Incerti in November 2016 at the FDANews Symposium in Bethesda, MD. Gilda's looks at US FDA Part 11 vs EU GMP Annex 11 and the quality driven data integrity approach. For more information on data integrity services, please visit www.pqe.eu
published: 03 Feb 2017 -

What's New and Getting Started in FCS Express 6 Clinical Edition
De Novo Software is proud to introduce FCS Express 6 Clinical Edition. FCS Express 6 Clinical Edition is the first third party flow cytometry data analysis software listed with the US FDA. FCS Express 6 combines ease of use with a host of powerful analysis and reporting features that are integral tools for clinical flow cytometry laboratories of any size.
published: 08 Apr 2017
Mobile Medical Apps & the US FDA Regulatory Oversight Still a Thin Red Line 20140914
- Order: Reorder
- Duration: 1:01:42
- Updated: 07 Feb 2015
- views: 21
US FDA Dr. Robert Califf Opens MDIC 2015 Annual Public Forum
- Order: Reorder
- Duration: 34:53
- Updated: 04 Nov 2015
- views: 70
- published: 04 Nov 2015
- views: 70
Band Baaja Bride show on NDTV Good Times (ft. Dr Chytra V Anand)
- Order: Reorder
- Duration: 45:19
- Updated: 21 Mar 2014
- views: 91270
- published: 21 Mar 2014
- views: 91270
HESI: November 17, 2014: DART Committee
- Order: Reorder
- Duration: 37:39
- Updated: 23 Apr 2015
- views: 26
2. Основы концепции биоэквивалентности
- Order: Reorder
- Duration: 30:07
- Updated: 12 Jul 2016
- views: 407
- published: 12 Jul 2016
- views: 407
Limecrime Velvetine Review with Check Ins (Pink Velvet) & Controversy
- Order: Reorder
- Duration: 35:15
- Updated: 30 Jun 2015
- views: 201972
- published: 30 Jun 2015
- views: 201972
Live Discussion on things and stuff: CBD, Coils, Modding, FDA
- Order: Reorder
- Duration: 1:05:55
- Updated: 21 Apr 2017
- views: 952
Band Baaja Bride show on NDTV Good Times (ft. Dr Chytra V Anand)
- Order: Reorder
- Duration: 37:55
- Updated: 15 Apr 2013
- views: 92071
- published: 15 Apr 2013
- views: 92071
PQE - QUALITY DRIVEN DATA INTEGRITY APPROACH
- Order: Reorder
- Duration: 47:32
- Updated: 03 Feb 2017
- views: 489
- published: 03 Feb 2017
- views: 489
What's New and Getting Started in FCS Express 6 Clinical Edition
- Order: Reorder
- Duration: 1:07:38
- Updated: 08 Apr 2017
- views: 73
- published: 08 Apr 2017
- views: 73


- Playlist
- Chat

Introduction to the US FDA
- Report rights infringement
- published: 05 Mar 2015
- views: 150

How Does the FDA Approve a Drug?
- Report rights infringement
- published: 20 Jul 2015
- views: 35249

FDA Inspection Do and Don't List
- Report rights infringement
- published: 30 May 2013
- views: 20067

Indian pharma under dark clouds of US FDA
- Report rights infringement
- published: 18 Mar 2016
- views: 136

U.S. FDA Food Facility Registration Renewal
- Report rights infringement
- published: 01 Aug 2016
- views: 177393

New U.S. FDA Food Labeling Rules
- Report rights infringement
- published: 16 Mar 2017
- views: 11

How Does The FDA Approve New Drugs?
- Report rights infringement
- published: 20 Jul 2015
- views: 68568

Brian G Nadel, Former Investigator, USFDA & Consultant || Pharma Regulators' Day || PhilipCapital
- Report rights infringement
- published: 22 Mar 2017
- views: 5816

Triclosan found in 75% of us, FDA knows it's dangerous
- Report rights infringement
- published: 11 Dec 2013
- views: 11998

US FDA Regulaciones de Etiquetado para Alimentos y Bebidas Español
- Report rights infringement
- published: 05 Jan 2015
- views: 40

- Playlist
- Chat

Have Questions About Traveling with Medication? The FDA Has Answers
- Report rights infringement
- published: 08 Feb 2016
- views: 0

12 Days in Europe in a Carry-On?! // Packing Challenge!
- Report rights infringement
- published: 13 Dec 2016
- views: 136312

Secrets of the FDA: The Business and Politics Behind the Food We Eat (2005)
- Report rights infringement
- published: 26 Jan 2017
- views: 364

Top 10 Travel Essentials | For the Airplane!
- Report rights infringement
- published: 20 Dec 2016
- views: 3754

Basic Guide to Exporting: Understanding of Free Sale Export Compliance
- Report rights infringement
- published: 19 Jun 2014
- views: 1539

Improving food safety at Jordan's hotels and restaurants
- Report rights infringement
- published: 20 Jan 2015
- views: 520

FDA CTP Webinar 6.26.2014
- Report rights infringement
- published: 24 Nov 2014
- views: 814

Nutrition Labels are Changing!
- Report rights infringement
- published: 21 May 2016
- views: 198

Final Rule for Sanitary Transportation
- Report rights infringement
- published: 12 May 2016
- views: 1960

Budget travel greece
- Report rights infringement
- published: 11 Oct 2014
- views: 9

- Playlist
- Chat

Introduction to the US FDA
- Report rights infringement
- published: 05 Mar 2015
- views: 150

How Does the FDA Approve a Drug?
- Report rights infringement
- published: 20 Jul 2015
- views: 35249

FDA Inspection Do and Don't List
- Report rights infringement
- published: 30 May 2013
- views: 20067

Indian pharma under dark clouds of US FDA
- Report rights infringement
- published: 18 Mar 2016
- views: 136

U.S. FDA Food Facility Registration Renewal
- Report rights infringement
- published: 01 Aug 2016
- views: 177393

New U.S. FDA Food Labeling Rules
- Report rights infringement
- published: 16 Mar 2017
- views: 11

How Does The FDA Approve New Drugs?
- Report rights infringement
- published: 20 Jul 2015
- views: 68568

Brian G Nadel, Former Investigator, USFDA & Consultant || Pharma Regulators' Day || PhilipCapital
- Report rights infringement
- published: 22 Mar 2017
- views: 5816

Triclosan found in 75% of us, FDA knows it's dangerous
- Report rights infringement
- published: 11 Dec 2013
- views: 11998

US FDA Regulaciones de Etiquetado para Alimentos y Bebidas Español
- Report rights infringement
- published: 05 Jan 2015
- views: 40

- Playlist
- Chat

Mobile Medical Apps & the US FDA Regulatory Oversight Still a Thin Red Line 20140914
- Report rights infringement
- published: 07 Feb 2015
- views: 21

US FDA Dr. Robert Califf Opens MDIC 2015 Annual Public Forum
- Report rights infringement
- published: 04 Nov 2015
- views: 70

Band Baaja Bride show on NDTV Good Times (ft. Dr Chytra V Anand)
- Report rights infringement
- published: 21 Mar 2014
- views: 91270

HESI: November 17, 2014: DART Committee
- Report rights infringement
- published: 23 Apr 2015
- views: 26

2. Основы концепции биоэквивалентности
- Report rights infringement
- published: 12 Jul 2016
- views: 407

Limecrime Velvetine Review with Check Ins (Pink Velvet) & Controversy
- Report rights infringement
- published: 30 Jun 2015
- views: 201972

Live Discussion on things and stuff: CBD, Coils, Modding, FDA
- Report rights infringement
- published: 21 Apr 2017
- views: 952

Band Baaja Bride show on NDTV Good Times (ft. Dr Chytra V Anand)
- Report rights infringement
- published: 15 Apr 2013
- views: 92071

PQE - QUALITY DRIVEN DATA INTEGRITY APPROACH
- Report rights infringement
- published: 03 Feb 2017
- views: 489

What's New and Getting Started in FCS Express 6 Clinical Edition
- Report rights infringement
- published: 08 Apr 2017
- views: 73
-
Lyrics list:lyrics
-
Standing At The Gates Of Failure, As We Fight
-
Slay The Firstborn, As We Fight
-
Resistance, As We Fight
-
Lost To The Madness, As We Fight
-
Evil Deeds, As We Fight
-
Left In Torment, As We Fight
-
Ignite The Fire, As We Fight
-
I Bury My Head In My Hands, As We Fight
-
Escaping The Poisoned Hands Of Despair, As We Fight
-
Embrace This Hell, As We Fight
-
-
-
-
-
-
-
-
-
-
-
-
-
-
Standing At The Gates Of Failure
Hateful/ lifeless/ as u pray for death.
At the gates of failure you see / yourself come to an end.
In times where life is war.
Can you afford to turn your back!
Just sit back and let it slide.
Watching life pass you by.
Was it all, like you pictured it.
Was it all you hoped it would be?
And I still have hope that we’ll someday be free.
Cause we are all here and we all bleed the same.
We all bleed the same!! (x2)
We all have hopes and dreams, and pre painted pictures, of how our life should be.
This could have been nothing.
We could have been nothing at all.
Nothing at all!
I will not settle for life of pain.
Because I’m so sick of this fucking place
There’s nothing left for me to say.
'A gift for the American bastards': North Korea's Kim fires back at Trump
Edit The Guardian 05 Jul 2017Ice block the size of Delaware about to break away from Antarctica as global temperatures rise, scientists warn
Edit The Independent 03 Jul 2017Qatar responds to Arab nations' demands before crisis meeting
Edit Irish Independent 05 Jul 2017Forty-Four States Refuse To Turn Over Voter Data For Trump Commission
Edit WorldNews.com 04 Jul 2017ISIS will lose Mosul and Raqqa. What happens next?
Edit The Washington Post 05 Jul 2017Global Recreational Vehicle (RV) Market - Industry Analysis, Size, Growth, Trends, and Forecast Report 2021
Edit Community news 05 Jul 2017US confirms North Korea's ICBM test
Edit Suryaa 05 Jul 2017Fossil Fuel Empire: A World of Vulnerability
Edit Resilience 05 Jul 2017North Korea crisis: US and South Korea fire missiles in show of force
Edit Stuff 05 Jul 2017Watch: US and South Korean military fire their own 'deep strike' missiles in show of ...
Edit TVNZ 05 Jul 2017US furious as North Korea claims its ICBM can carry ‘large, heavy nuclear warhead’
Edit India TV 05 Jul 2017Ban on gadgets lifted for Emirates, Turkish Airlines flights to US
Edit Deccan Herald 05 Jul 2017Laptop ban lifted on Emirates flights
Edit Graphic 05 Jul 2017Ban on gadgets lifted for Emirates, Turkish Airlines flights
Edit DNA India 05 Jul 2017Trump, Putin to hold bilateral meeting at G20 summit: White House
Edit Yahoo Daily News 05 Jul 2017US, S. Korea stage missile drills after North's test
Edit Yahoo Daily News 05 Jul 2017Electronics Ban Lifted By US On Emirates' Flights From Dubai
Edit Suryaa 05 Jul 2017US lifts electronics ban on Emirates' flights from Dubai
Edit Yahoo Daily News 05 Jul 2017US offer of Sea Guardian drones to India signals converging strategic interests
Edit Hindustan Times 05 Jul 2017- 1
- 2
- 3
- 4
- 5
- Next page »




























