- published: 16 Dec 2015
- views: 68236
-
remove the playlistThermodynamic Free Energy
-
remove the playlistLatest Videos
-
remove the playlistLongest Videos
- remove the playlistThermodynamic Free Energy
- remove the playlistLatest Videos
- remove the playlistLongest Videos
- published: 18 Jul 2016
- views: 1989
- published: 13 Jun 2017
- views: 5271
- published: 28 Sep 2009
- views: 313264
- published: 14 Jan 2014
- views: 141989
- published: 02 Jul 2013
- views: 645804
- published: 30 Jun 2011
- views: 385779
- published: 22 Jun 2017
- views: 191
- published: 09 Dec 2012
- views: 128609

Thermodynamic free energy
The thermodynamic free energy is the amount of work that a thermodynamic system can perform. The concept is useful in the thermodynamics of chemical or thermal processes in engineering and science. The free energy is the internal energy of a system minus the amount of energy that cannot be used to perform work. This unusable energy is given by the entropy of a system multiplied by the temperature of the system.
Like the internal energy, the free energy is a thermodynamic state function. Energy is a generalization of free energy, since energy is the ability to do work which is actually free energy.
Overview
Free energy is that portion of any first-law energy that is available to perform thermodynamic work; i.e., work mediated by thermal energy. Free energy is subject to irreversible loss in the course of such work. Since first-law energy is always conserved, it is evident that free energy is an expendable, second-law kind of energy that can perform work within finite amounts of time. Several free energy functions may be formulated based on system criteria. Free energy functions are Legendre transformations of the internal energy. For processes involving a system at constant pressure p and temperature T, the Gibbs free energy is the most useful because, in addition to subsuming any entropy change due merely to heat, it does the same for the pdV work needed to "make space for additional molecules" produced by various processes. (Hence its utility to solution-phase chemists, including biochemists.) The Helmholtz free energy has a special theoretical importance since it is proportional to the logarithm of the partition function for the canonical ensemble in statistical mechanics. (Hence its utility to physicists; and to gas-phase chemists and engineers, who do not want to ignore pdV work.)
This article is licensed under the Creative Commons Attribution-ShareAlike 3.0 Unported License, which means that you can copy and modify it as long as the entire work (including additions) remains under this license.
Free energy
Free energy may refer to:
In science:
- Helmholtz free energy (A=U–TS), the energy that can be converted into work at a constant temperature and volume
- Work content, a related concept used in chemistry
- Gibbs free energy (G=H–TS), the energy that can be converted into work at a constant temperature and pressure throughout a system
- Work content, a related concept used in chemistry
In economics:
This article is licensed under the Creative Commons Attribution-ShareAlike 3.0 Unported License, which means that you can copy and modify it as long as the entire work (including additions) remains under this license.

Gibbs free energy
In thermodynamics, the Gibbs free energy (IUPAC recommended name: Gibbs energy or Gibbs function; also known as free enthalpy to distinguish it from Helmholtz free energy) is a thermodynamic potential that measures the maximum or reversible work that may be performed by a thermodynamic system at a constant temperature and pressure (isothermal, isobaric). Just as in mechanics, where potential energy is defined as capacity to do work, similarly different potentials have different meanings. The Gibbs free energy (kJ in SI units) is the maximum amount of non-expansion work that can be extracted from a thermodynamically closed system (one that can exchange heat and work with its surroundings, but not matter); this maximum can be attained only in a completely reversible process. When a system changes from a well-defined initial state to a well-defined final state, the Gibbs free energy change ΔG equals the work exchanged by the system with its surroundings, minus the work of the pressure forces, during a reversible transformation of the system from the initial state to the final state.
This article is licensed under the Creative Commons Attribution-ShareAlike 3.0 Unported License, which means that you can copy and modify it as long as the entire work (including additions) remains under this license.

Khan Academy
Khan Academy is a non-profit educational organization created in 2006 by educator Salman Khan with the aim of providing a free, world-class education for anyone, anywhere. The organization produces short lectures in the form of YouTube videos. In addition to micro lectures, the organization's website features practice exercises and tools for educators. All resources are available for free to anyone around the world. The main language of the website is English, but the content is also available in other languages.
History
The founder of the organization, Salman Khan, was born in New Orleans, Louisiana, United States to immigrant parents from Bangladesh and India. After earning three degrees from the Massachusetts Institute of Technology (a BS in mathematics, a BS in electrical engineering and computer science, and an MEng in electrical engineering and computer science), he pursued an MBA from Harvard Business School.
In late 2004, Khan began tutoring his cousin Nadia who needed help with math using Yahoo!'s Doodle notepad.When other relatives and friends sought similar help, he decided that it would be more practical to distribute the tutorials on YouTube. The videos' popularity and the testimonials of appreciative students prompted Khan to quit his job in finance as a hedge fund analyst at Connective Capital Management in 2009, and focus on the tutorials (then released under the moniker "Khan Academy") full-time.
This article is licensed under the Creative Commons Attribution-ShareAlike 3.0 Unported License, which means that you can copy and modify it as long as the entire work (including additions) remains under this license.

Laws of thermodynamics
The four laws of thermodynamics define fundamental physical quantities (temperature, energy, and entropy) that characterize thermodynamic systems. The laws describe how these quantities behave under various circumstances, and forbid certain phenomena (such as perpetual motion).
The four laws of thermodynamics are:
This article is licensed under the Creative Commons Attribution-ShareAlike 3.0 Unported License, which means that you can copy and modify it as long as the entire work (including additions) remains under this license.
- Loading...

-
 8:12
8:12The Laws of Thermodynamics, Entropy, and Gibbs Free Energy
The Laws of Thermodynamics, Entropy, and Gibbs Free EnergyThe Laws of Thermodynamics, Entropy, and Gibbs Free Energy
We've all heard of the Laws of Thermodynamics, but what are they really? What the heck is entropy and what does it mean for the fate of the universe? How does soap work?! So many questions answered in this clip! Enjoy! Subscribe: http://bit.ly/ProfDaveSubscribe ProfessorDaveExplains@gmail.com http://professordaveexplains.com http://facebook.com/ProfessorDaveExpl... http://twitter.com/DaveExplains General Chemistry - Online Tutorials: http://bit.ly/ProfDaveGenChem Organic Chemistry - Online Tutorials: http://bit.ly/ProfDaveOrgChem Science for Common Folk - Online Tutorials: http://bit.ly/ProfDaveScience4CommonFolk -
 53:58
53:58Gibbs Free Energy - Equilibrium Constant, Enthalpy & Entropy - Equations & Practice Problems
Gibbs Free Energy - Equilibrium Constant, Enthalpy & Entropy - Equations & Practice ProblemsGibbs Free Energy - Equilibrium Constant, Enthalpy & Entropy - Equations & Practice Problems
This chemistry video tutorial provides a lecture review on gibbs free energy, the equilibrium constant K, enthalpy and entropy. it provides a list of equations and formulas as well as the appropriate units. It contains plenty of examples and practice problems. Here is a list of topics: 1. Entropy Definition - Concepts and Examples 2. Entropy of Solids, Liquids, and Gases 3. How To Determine / Predict The Sign of the Entropy Change / Delta S for a reaction 4. Second Law of Thermodynamics - The Entropy of Universe, System and Surroundings 5. Delta G, H, T and S equation 6. Enthalpy - Heat Exchange at Constant Pressure - Endothermic and Exothermic Reactions 7. Gibbs Free Energy and the ability to do useful work 8. Spontaneity - Spontaneous and Nonspontaneous Processes 9. Delta G = 0, Reversible Process at Equilibrium 10. How To Calculate Delta G Naught Using the Equilibrium Constant K 11. Entropy of Reaction = Products - Reactants 12. Entropy = q/T heat absorbed for a reversible reaction and temperature 13. The relationship between temperature and entropy 14. Nonstandard Delta G calculations 15. Delta G - Gibbs Free Energy and Le Chatelier's Principle 16. Delta G Table / Chart - How To Determine if a Reaction is Spontaneous at Low or High Temperatures, Always Spontaneous or Nonspontaneous based on the signs of enthalpy and entropy -
 5:02
5:02Free Energy Light Bulbs
Free Energy Light BulbsFree Energy Light Bulbs
Free Energy Generator For Light Bulb http://youtube.com/tachnicalinfohindi http://kodayworld.blogspot.com Thanks for watching video Like and share video link subscribe channel Technical info This video Entertainment , Fun , Magic , Puzzle -
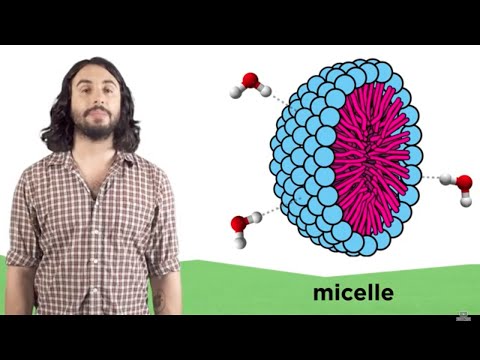
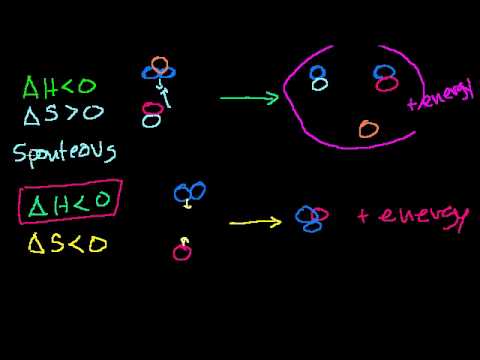 17:40
17:40Gibbs free energy and spontaneity | Chemistry | Khan Academy
Gibbs free energy and spontaneity | Chemistry | Khan AcademyGibbs free energy and spontaneity | Chemistry | Khan Academy
Intuition behind why spontaneity is driven by enthalpy, entropy and temperature. Introduction to Gibbs free energy. Created by Sal Khan. Watch the next lesson: https://www.khanacademy.org/science/chemistry/thermodynamics-chemistry/gibbs-free-energy/v/gibbs-free-energy-example?utm_source=YT&utm;_medium=Desc&utm;_campaign=chemistry Missed the previous lesson? https://www.khanacademy.org/science/chemistry/thermodynamics-chemistry/entropy-chemistry-sal/v/maxwell-s-demon?utm_source=YT&utm;_medium=Desc&utm;_campaign=chemistry Chemistry on Khan Academy: Did you know that everything is made out of chemicals? Chemistry is the study of matter: its composition, properties, and reactivity. This material roughly covers a first-year high school or college course, and a good understanding of algebra is helpful. About Khan Academy: Khan Academy offers practice exercises, instructional videos, and a personalized learning dashboard that empower learners to study at their own pace in and outside of the classroom. We tackle math, science, computer programming, history, art history, economics, and more. Our math missions guide learners from kindergarten to calculus using state-of-the-art, adaptive technology that identifies strengths and learning gaps. We've also partnered with institutions like NASA, The Museum of Modern Art, The California Academy of Sciences, and MIT to offer specialized content. For free. For everyone. Forever. #YouCanLearnAnything Subscribe to Khan Academy’s Chemistry channel: https://www.youtube.com/channel/UCyEot66LrwWFEMONvrIBh3A?sub_confirmation=1 Subscribe to Khan Academy: https://www.youtube.com/subscription_center?add_user=khanacademy -
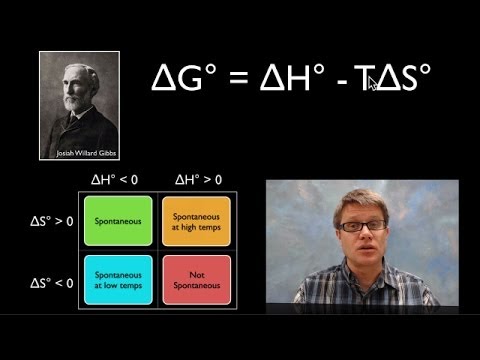 7:57
7:57Using Gibbs Free Energy
Using Gibbs Free EnergyUsing Gibbs Free Energy
059 - Using Gibbs Free Energy In this video Paul Andersen explains how you can use the Gibbs Free Energy equation to determine if a process is spontaneous or not spontaneous. If the ΔG is less than zero the process is spontaneous. If the ΔG is greater than zero the process is not spontaneous. If the ΔG is equal to zero the process is at equilibrium. The ΔH, ΔS, and T are all used to calculate ΔG. Do you speak another language? Help me translate my videos: http://www.bozemanscience.com/translations/ Music Attribution Title: String Theory Artist: Herman Jolly http://sunsetvalley.bandcamp.com/track/string-theory All of the images are licensed under creative commons and public domain licensing: "File:Hex ice.GIF." Wikipedia, the Free Encyclopedia. Accessed December 29, 2013. http://en.wikipedia.org/wiki/File:Hex_ice.GIF. "File:Josiah Willard Gibbs -from MMS-.jpg." Wikipedia, the Free Encyclopedia. Accessed December 29, 2013. http://en.wikipedia.org/wiki/File:Josiah_Willard_Gibbs_-from_MMS-.jpg. "File:ThermiteReaction.jpg." Wikipedia, the Free Encyclopedia, December 11, 2013. http://en.wikipedia.org/w/index.php?title=File:ThermiteReaction.jpg&oldid;=543297076. Gkai. English: Erlenmeyer Flask, May 23, 2010. Own work. http://commons.wikimedia.org/wiki/File:Erlenmeyer-flask.svg. Haacken, User: Herbert. English: Instant Cold Pack, March 15, 2012. Own work: Herbert Haacken. http://commons.wikimedia.org/wiki/File:2012-03-15_Ruck-Zuck-Pack_K%C3%A4lte_Katalog_Small.gif. Splettstoesser, Thomas. Hydrogen Bonds in Liquid Water Molecular Dynamics Simulation (Tip3P Water Model with CHARMM Force Field), June 25, 2007. self-made with open source visualization software PyMol. http://commons.wikimedia.org/wiki/File:Liquid_water_hydrogen_bond.png. -
 13:41
13:41Entropy: Embrace the Chaos! Crash Course Chemistry #20
Entropy: Embrace the Chaos! Crash Course Chemistry #20Entropy: Embrace the Chaos! Crash Course Chemistry #20
Life is chaos and the universe tends toward disorder. But why? If you think about it, there are only a few ways for things to be arranged in an organized manner, but there are nearly infinite other ways for those same things to be arranged. Simple rules of probability dictate that it's much more likely for stuff to be in one of the many disorganized states than in one of the few organized states. This tendency is so unavoidable that it's known as the 2nd Law of Thermodynamics. Obviously, disorder is a pretty big deal in the universe and that makes it a pretty big deal in chemistry - it's such a big deal that scientists have a special name for it: entropy. In chemistry, entropy is the measure of molecular randomness, or disorder. For the next thirteen minutes, Hank hopes you will embrace the chaos as he teaches you about entropy. -- Table of Contents Second Law of Thermodynamics :45 Entropy 2:01 DEMONSTRATION! 4:28 BA(OH)2•8H2O+NH4Ci 10:25 J.W. Gibbs & Gibbs Free Energy 7:23 -- Want to find Crash Course elsewhere on the internet? Facebook - http://www.facebook.com/YouTubeCrashC... Twitter - http://www.twitter.com/TheCrashCourse Tumblr - http://thecrashcourse.tumblr.com Support CrashCourse on Subbable: http://subbable.com/crashcourse -
 13:00
13:00Gibbs Free Energy
Gibbs Free EnergyGibbs Free Energy
Paul Andersen attempts to explain Gibbs Free Energy. He begins by using three spontaneous reactions to explain how a change in enthalpy, entropy and temperature can affect the free energy of a system. He then applies this concept to cellular respiration and photosynthesis. Intro Music Atribution Title: I4dsong_loop_main.wav Artist: CosmicD Link to sound: http://www.freesound.org/people/CosmicD/sounds/72556/ Creative Commons Atribution License -
 1:26
1:26Thermodynamic Free Energy
Thermodynamic Free EnergyThermodynamic Free Energy
-
 7:24
7:24Ac Generator 220 volt 100 Watt free energy Part 2 with strong proof.... ( Et Discover)
Ac Generator 220 volt 100 Watt free energy Part 2 with strong proof.... ( Et Discover)Ac Generator 220 volt 100 Watt free energy Part 2 with strong proof.... ( Et Discover)
how to make 100 Watt inverter .. 1= 2 motor 12 volt... 2= n3055 mosfet 2 3= 10 e resistor 2 4= 120 E resistor 2 5= ac Fan regulator 4= diode bridge rectifier 5= 3300 mfd 25 volt capacitor 6= veroboard 7= a 4 volt battery 8= A ups transformer.. 9= on off swtch part 1 video link== https://www.youtube.com/watch?v=dmlwGDNQUHw -
 13:50
13:50Second Law of Thermodynamics,Entropy &Gibbs; Free Energy
Second Law of Thermodynamics,Entropy &Gibbs; Free EnergySecond Law of Thermodynamics,Entropy &Gibbs; Free Energy
Here is a lecture to understand 2nd law of thermodynamics in a conceptual way. Along with 2nd law, concepts of entropy and Gibbs free energy are also explained. Check http://www.learnengineering.org/2012/12/understanding-second-law-of.html to get to know about industrial applications of second law of thermodynamics.
-

The Laws of Thermodynamics, Entropy, and Gibbs Free Energy
We've all heard of the Laws of Thermodynamics, but what are they really? What the heck is entropy and what does it mean for the fate of the universe? How does soap work?! So many questions answered in this clip! Enjoy! Subscribe: http://bit.ly/ProfDaveSubscribe ProfessorDaveExplains@gmail.com http://professordaveexplains.com http://facebook.com/ProfessorDaveExpl... http://twitter.com/DaveExplains General Chemistry - Online Tutorials: http://bit.ly/ProfDaveGenChem Organic Chemistry - Online Tutorials: http://bit.ly/ProfDaveOrgChem Science for Common Folk - Online Tutorials: http://bit.ly/ProfDaveScience4CommonFolk
published: 16 Dec 2015 -

Gibbs Free Energy - Equilibrium Constant, Enthalpy & Entropy - Equations & Practice Problems
This chemistry video tutorial provides a lecture review on gibbs free energy, the equilibrium constant K, enthalpy and entropy. it provides a list of equations and formulas as well as the appropriate units. It contains plenty of examples and practice problems. Here is a list of topics: 1. Entropy Definition - Concepts and Examples 2. Entropy of Solids, Liquids, and Gases 3. How To Determine / Predict The Sign of the Entropy Change / Delta S for a reaction 4. Second Law of Thermodynamics - The Entropy of Universe, System and Surroundings 5. Delta G, H, T and S equation 6. Enthalpy - Heat Exchange at Constant Pressure - Endothermic and Exothermic Reactions 7. Gibbs Free Energy and the ability to do useful work 8. Spontaneity - Spontaneous and Nonspontaneous Processes 9. Delta G...
published: 18 Jul 2016 -

Free Energy Light Bulbs
Free Energy Generator For Light Bulb http://youtube.com/tachnicalinfohindi http://kodayworld.blogspot.com Thanks for watching video Like and share video link subscribe channel Technical info This video Entertainment , Fun , Magic , Puzzle
published: 13 Jun 2017 -
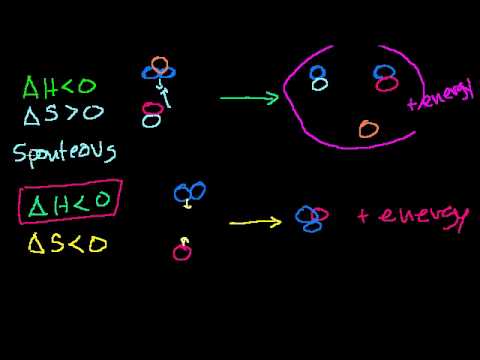
Gibbs free energy and spontaneity | Chemistry | Khan Academy
Intuition behind why spontaneity is driven by enthalpy, entropy and temperature. Introduction to Gibbs free energy. Created by Sal Khan. Watch the next lesson: https://www.khanacademy.org/science/chemistry/thermodynamics-chemistry/gibbs-free-energy/v/gibbs-free-energy-example?utm_source=YT&utm;_medium=Desc&utm;_campaign=chemistry Missed the previous lesson? https://www.khanacademy.org/science/chemistry/thermodynamics-chemistry/entropy-chemistry-sal/v/maxwell-s-demon?utm_source=YT&utm;_medium=Desc&utm;_campaign=chemistry Chemistry on Khan Academy: Did you know that everything is made out of chemicals? Chemistry is the study of matter: its composition, properties, and reactivity. This material roughly covers a first-year high school or college course, and a good understanding of algebra is he...
published: 28 Sep 2009 -

Using Gibbs Free Energy
059 - Using Gibbs Free Energy In this video Paul Andersen explains how you can use the Gibbs Free Energy equation to determine if a process is spontaneous or not spontaneous. If the ΔG is less than zero the process is spontaneous. If the ΔG is greater than zero the process is not spontaneous. If the ΔG is equal to zero the process is at equilibrium. The ΔH, ΔS, and T are all used to calculate ΔG. Do you speak another language? Help me translate my videos: http://www.bozemanscience.com/translations/ Music Attribution Title: String Theory Artist: Herman Jolly http://sunsetvalley.bandcamp.com/track/string-theory All of the images are licensed under creative commons and public domain licensing: "File:Hex ice.GIF." Wikipedia, the Free Encyclopedia. Accessed December 29, 2013. http://en...
published: 14 Jan 2014 -

Entropy: Embrace the Chaos! Crash Course Chemistry #20
Life is chaos and the universe tends toward disorder. But why? If you think about it, there are only a few ways for things to be arranged in an organized manner, but there are nearly infinite other ways for those same things to be arranged. Simple rules of probability dictate that it's much more likely for stuff to be in one of the many disorganized states than in one of the few organized states. This tendency is so unavoidable that it's known as the 2nd Law of Thermodynamics. Obviously, disorder is a pretty big deal in the universe and that makes it a pretty big deal in chemistry - it's such a big deal that scientists have a special name for it: entropy. In chemistry, entropy is the measure of molecular randomness, or disorder. For the next thirteen minutes, Hank hopes you will embrace th...
published: 02 Jul 2013 -

Gibbs Free Energy
Paul Andersen attempts to explain Gibbs Free Energy. He begins by using three spontaneous reactions to explain how a change in enthalpy, entropy and temperature can affect the free energy of a system. He then applies this concept to cellular respiration and photosynthesis. Intro Music Atribution Title: I4dsong_loop_main.wav Artist: CosmicD Link to sound: http://www.freesound.org/people/CosmicD/sounds/72556/ Creative Commons Atribution License
published: 30 Jun 2011 -

Thermodynamic Free Energy
published: 02 Nov 2011 -

Ac Generator 220 volt 100 Watt free energy Part 2 with strong proof.... ( Et Discover)
how to make 100 Watt inverter .. 1= 2 motor 12 volt... 2= n3055 mosfet 2 3= 10 e resistor 2 4= 120 E resistor 2 5= ac Fan regulator 4= diode bridge rectifier 5= 3300 mfd 25 volt capacitor 6= veroboard 7= a 4 volt battery 8= A ups transformer.. 9= on off swtch part 1 video link== https://www.youtube.com/watch?v=dmlwGDNQUHw
published: 22 Jun 2017 -

Second Law of Thermodynamics,Entropy &Gibbs; Free Energy
Here is a lecture to understand 2nd law of thermodynamics in a conceptual way. Along with 2nd law, concepts of entropy and Gibbs free energy are also explained. Check http://www.learnengineering.org/2012/12/understanding-second-law-of.html to get to know about industrial applications of second law of thermodynamics.
published: 09 Dec 2012
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy
- Order: Reorder
- Duration: 8:12
- Updated: 16 Dec 2015
- views: 68236
- published: 16 Dec 2015
- views: 68236
Gibbs Free Energy - Equilibrium Constant, Enthalpy & Entropy - Equations & Practice Problems
- Order: Reorder
- Duration: 53:58
- Updated: 18 Jul 2016
- views: 1989
- published: 18 Jul 2016
- views: 1989
Free Energy Light Bulbs
- Order: Reorder
- Duration: 5:02
- Updated: 13 Jun 2017
- views: 5271
- published: 13 Jun 2017
- views: 5271
Gibbs free energy and spontaneity | Chemistry | Khan Academy
- Order: Reorder
- Duration: 17:40
- Updated: 28 Sep 2009
- views: 313264
- published: 28 Sep 2009
- views: 313264
Using Gibbs Free Energy
- Order: Reorder
- Duration: 7:57
- Updated: 14 Jan 2014
- views: 141989
- published: 14 Jan 2014
- views: 141989
Entropy: Embrace the Chaos! Crash Course Chemistry #20
- Order: Reorder
- Duration: 13:41
- Updated: 02 Jul 2013
- views: 645804
- published: 02 Jul 2013
- views: 645804
Gibbs Free Energy
- Order: Reorder
- Duration: 13:00
- Updated: 30 Jun 2011
- views: 385779
- published: 30 Jun 2011
- views: 385779
Thermodynamic Free Energy
- Order: Reorder
- Duration: 1:26
- Updated: 02 Nov 2011
- views: 5161
- published: 02 Nov 2011
- views: 5161
Ac Generator 220 volt 100 Watt free energy Part 2 with strong proof.... ( Et Discover)
- Order: Reorder
- Duration: 7:24
- Updated: 22 Jun 2017
- views: 191
- published: 22 Jun 2017
- views: 191
Second Law of Thermodynamics,Entropy &Gibbs; Free Energy
- Order: Reorder
- Duration: 13:50
- Updated: 09 Dec 2012
- views: 128609
- published: 09 Dec 2012
- views: 128609
-

The Laws of Thermodynamics, Entropy, and Gibbs Free Energy
We've all heard of the Laws of Thermodynamics, but what are they really? What the heck is entropy and what does it mean for the fate of the universe? How does soap work?! So many questions answered in this clip! Enjoy! Subscribe: http://bit.ly/ProfDaveSubscribe ProfessorDaveExplains@gmail.com http://professordaveexplains.com http://facebook.com/ProfessorDaveExpl... http://twitter.com/DaveExplains General Chemistry - Online Tutorials: http://bit.ly/ProfDaveGenChem Organic Chemistry - Online Tutorials: http://bit.ly/ProfDaveOrgChem Science for Common Folk - Online Tutorials: http://bit.ly/ProfDaveScience4CommonFolk
published: 16 Dec 2015 -

Gibbs Free Energy - Equilibrium Constant, Enthalpy & Entropy - Equations & Practice Problems
This chemistry video tutorial provides a lecture review on gibbs free energy, the equilibrium constant K, enthalpy and entropy. it provides a list of equations and formulas as well as the appropriate units. It contains plenty of examples and practice problems. Here is a list of topics: 1. Entropy Definition - Concepts and Examples 2. Entropy of Solids, Liquids, and Gases 3. How To Determine / Predict The Sign of the Entropy Change / Delta S for a reaction 4. Second Law of Thermodynamics - The Entropy of Universe, System and Surroundings 5. Delta G, H, T and S equation 6. Enthalpy - Heat Exchange at Constant Pressure - Endothermic and Exothermic Reactions 7. Gibbs Free Energy and the ability to do useful work 8. Spontaneity - Spontaneous and Nonspontaneous Processes 9. Delta G...
published: 18 Jul 2016 -

Free Energy Light Bulbs
Free Energy Generator For Light Bulb http://youtube.com/tachnicalinfohindi http://kodayworld.blogspot.com Thanks for watching video Like and share video link subscribe channel Technical info This video Entertainment , Fun , Magic , Puzzle
published: 13 Jun 2017 -
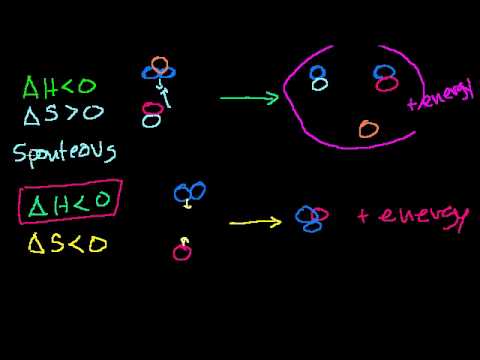
Gibbs free energy and spontaneity | Chemistry | Khan Academy
Intuition behind why spontaneity is driven by enthalpy, entropy and temperature. Introduction to Gibbs free energy. Created by Sal Khan. Watch the next lesson: https://www.khanacademy.org/science/chemistry/thermodynamics-chemistry/gibbs-free-energy/v/gibbs-free-energy-example?utm_source=YT&utm;_medium=Desc&utm;_campaign=chemistry Missed the previous lesson? https://www.khanacademy.org/science/chemistry/thermodynamics-chemistry/entropy-chemistry-sal/v/maxwell-s-demon?utm_source=YT&utm;_medium=Desc&utm;_campaign=chemistry Chemistry on Khan Academy: Did you know that everything is made out of chemicals? Chemistry is the study of matter: its composition, properties, and reactivity. This material roughly covers a first-year high school or college course, and a good understanding of algebra is he...
published: 28 Sep 2009 -

Using Gibbs Free Energy
059 - Using Gibbs Free Energy In this video Paul Andersen explains how you can use the Gibbs Free Energy equation to determine if a process is spontaneous or not spontaneous. If the ΔG is less than zero the process is spontaneous. If the ΔG is greater than zero the process is not spontaneous. If the ΔG is equal to zero the process is at equilibrium. The ΔH, ΔS, and T are all used to calculate ΔG. Do you speak another language? Help me translate my videos: http://www.bozemanscience.com/translations/ Music Attribution Title: String Theory Artist: Herman Jolly http://sunsetvalley.bandcamp.com/track/string-theory All of the images are licensed under creative commons and public domain licensing: "File:Hex ice.GIF." Wikipedia, the Free Encyclopedia. Accessed December 29, 2013. http://en...
published: 14 Jan 2014 -

Entropy: Embrace the Chaos! Crash Course Chemistry #20
Life is chaos and the universe tends toward disorder. But why? If you think about it, there are only a few ways for things to be arranged in an organized manner, but there are nearly infinite other ways for those same things to be arranged. Simple rules of probability dictate that it's much more likely for stuff to be in one of the many disorganized states than in one of the few organized states. This tendency is so unavoidable that it's known as the 2nd Law of Thermodynamics. Obviously, disorder is a pretty big deal in the universe and that makes it a pretty big deal in chemistry - it's such a big deal that scientists have a special name for it: entropy. In chemistry, entropy is the measure of molecular randomness, or disorder. For the next thirteen minutes, Hank hopes you will embrace th...
published: 02 Jul 2013 -

Gibbs Free Energy
Paul Andersen attempts to explain Gibbs Free Energy. He begins by using three spontaneous reactions to explain how a change in enthalpy, entropy and temperature can affect the free energy of a system. He then applies this concept to cellular respiration and photosynthesis. Intro Music Atribution Title: I4dsong_loop_main.wav Artist: CosmicD Link to sound: http://www.freesound.org/people/CosmicD/sounds/72556/ Creative Commons Atribution License
published: 30 Jun 2011 -

Thermodynamic Free Energy
published: 02 Nov 2011 -

Ac Generator 220 volt 100 Watt free energy Part 2 with strong proof.... ( Et Discover)
how to make 100 Watt inverter .. 1= 2 motor 12 volt... 2= n3055 mosfet 2 3= 10 e resistor 2 4= 120 E resistor 2 5= ac Fan regulator 4= diode bridge rectifier 5= 3300 mfd 25 volt capacitor 6= veroboard 7= a 4 volt battery 8= A ups transformer.. 9= on off swtch part 1 video link== https://www.youtube.com/watch?v=dmlwGDNQUHw
published: 22 Jun 2017 -

Second Law of Thermodynamics,Entropy &Gibbs; Free Energy
Here is a lecture to understand 2nd law of thermodynamics in a conceptual way. Along with 2nd law, concepts of entropy and Gibbs free energy are also explained. Check http://www.learnengineering.org/2012/12/understanding-second-law-of.html to get to know about industrial applications of second law of thermodynamics.
published: 09 Dec 2012
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy
- Order: Reorder
- Duration: 8:12
- Updated: 16 Dec 2015
- views: 68236
- published: 16 Dec 2015
- views: 68236
Gibbs Free Energy - Equilibrium Constant, Enthalpy & Entropy - Equations & Practice Problems
- Order: Reorder
- Duration: 53:58
- Updated: 18 Jul 2016
- views: 1989
- published: 18 Jul 2016
- views: 1989
Free Energy Light Bulbs
- Order: Reorder
- Duration: 5:02
- Updated: 13 Jun 2017
- views: 5271
- published: 13 Jun 2017
- views: 5271
Gibbs free energy and spontaneity | Chemistry | Khan Academy
- Order: Reorder
- Duration: 17:40
- Updated: 28 Sep 2009
- views: 313264
- published: 28 Sep 2009
- views: 313264
Using Gibbs Free Energy
- Order: Reorder
- Duration: 7:57
- Updated: 14 Jan 2014
- views: 141989
- published: 14 Jan 2014
- views: 141989
Entropy: Embrace the Chaos! Crash Course Chemistry #20
- Order: Reorder
- Duration: 13:41
- Updated: 02 Jul 2013
- views: 645804
- published: 02 Jul 2013
- views: 645804
Gibbs Free Energy
- Order: Reorder
- Duration: 13:00
- Updated: 30 Jun 2011
- views: 385779
- published: 30 Jun 2011
- views: 385779
Thermodynamic Free Energy
- Order: Reorder
- Duration: 1:26
- Updated: 02 Nov 2011
- views: 5161
- published: 02 Nov 2011
- views: 5161
Ac Generator 220 volt 100 Watt free energy Part 2 with strong proof.... ( Et Discover)
- Order: Reorder
- Duration: 7:24
- Updated: 22 Jun 2017
- views: 191
- published: 22 Jun 2017
- views: 191
Second Law of Thermodynamics,Entropy &Gibbs; Free Energy
- Order: Reorder
- Duration: 13:50
- Updated: 09 Dec 2012
- views: 128609
- published: 09 Dec 2012
- views: 128609
-

Gibbs Free Energy - Equilibrium Constant, Enthalpy & Entropy - Equations & Practice Problems
This chemistry video tutorial provides a lecture review on gibbs free energy, the equilibrium constant K, enthalpy and entropy. it provides a list of equations and formulas as well as the appropriate units. It contains plenty of examples and practice problems. Here is a list of topics: 1. Entropy Definition - Concepts and Examples 2. Entropy of Solids, Liquids, and Gases 3. How To Determine / Predict The Sign of the Entropy Change / Delta S for a reaction 4. Second Law of Thermodynamics - The Entropy of Universe, System and Surroundings 5. Delta G, H, T and S equation 6. Enthalpy - Heat Exchange at Constant Pressure - Endothermic and Exothermic Reactions 7. Gibbs Free Energy and the ability to do useful work 8. Spontaneity - Spontaneous and Nonspontaneous Processes 9. Delta G...
published: 18 Jul 2016 -

Thermodynamics 37 : Gibbs Helmholtz Free Energies
In this video I continue with my series of tutorial videos on Thermal Physics and Thermodynamics. It's pitched at undergraduate level and while it is mainly aimed at physics majors, it should be useful to anybody taking a first course in thermodynamics such as engineers etc.. The course covers topics such as the Ideal Gas Law, Entropy, Enthalpy, Gibbs' and Helmholtz' Free Energy, Heat Capacity, Einstein Solids, Taylor and MacLaurin Series / Expansions, phase transformations, thermodynamics identities, the Clausius Clapeyron Relation, Joule Thompson Throttling, Adiabatic Cooling, the paramagnet and of course the all important Laws of Thermodynamics Thank you for watching and I hope that this matches your requirements. Please feel free to provide feedback via comments and share with your ...
published: 01 Sep 2013 -

Thermodynamics-Gibbs Free Energy
This screencast has been created with Explain Everything™ Interactive Whiteboard for iPad
published: 31 Mar 2016 -

Thermodynamics, Entropy, Enthalpy & Gibbs Free Energy
A brief discussion regarding these concepts and how they apply to chemical reactions.
published: 21 Dec 2013 -

03 CBSE Class XI Chemistry Thermodynamics Entropy and Gibbs Energy
published: 06 Oct 2012 -

Dennis Lee - Free Energy Demonstration - Unconvincing
Dennis Lee - Free Energy Demonstration - Unconvincing free enery playlist is at : https://www.youtube.com/playlist?list=PLF299B85160E140C7
published: 06 Oct 2015 -

Free Energy, the embarrassing Truth
published: 23 Dec 2014 -

The Searl Effect, Free Energy Generator - Documentary
The Searl Effect was discovered by John Roy Robert Searl in 1946. Put simply, it is a method of extracting clean and sustainable electrical energy. The SEG consists of three fixed stator rings that are uniquely magnetized with patterns setup to generate continual motion of similarly magnetized cylindrical rotors. The magnetic rotors or rollers consist of eight segmented components made of the same four layers of concentric materials that make up the stators. The rollers have both freedom of spin and rotation around the stator which generates both mechanical and electrical power. The SEG is an 'open system' of energy conversion that is in accordance with known thermodynamic laws; particularly as it may apply at the quantum level. The open energy cycle of the SEG enables it to function bot...
published: 29 May 2017 -

Introduction to Free-Energy Calculations - Chris Chipot
Free Energy Methods, MDFF NBCR & TCBG Training Program: Simulation-Based Drug Discovery September 21, 2015 to September 25, 2015 University of California, San Diego
published: 14 Oct 2015 -

Gibbs Free Energy - Equilibrium Constant, Enthalpy & Entropy - Equations & Practice Problems
- Order: Reorder
- Duration: 53:58
- Updated: 18 Jul 2016
- views: 1989
- published: 18 Jul 2016
- views: 1989
Thermodynamics 37 : Gibbs Helmholtz Free Energies
- Order: Reorder
- Duration: 22:52
- Updated: 01 Sep 2013
- views: 13757
- published: 01 Sep 2013
- views: 13757
Thermodynamics-Gibbs Free Energy
- Order: Reorder
- Duration: 25:51
- Updated: 31 Mar 2016
- views: 456
- published: 31 Mar 2016
- views: 456
Thermodynamics, Entropy, Enthalpy & Gibbs Free Energy
- Order: Reorder
- Duration: 24:27
- Updated: 21 Dec 2013
- views: 20990
- published: 21 Dec 2013
- views: 20990
03 CBSE Class XI Chemistry Thermodynamics Entropy and Gibbs Energy
- Order: Reorder
- Duration: 26:41
- Updated: 06 Oct 2012
- views: 13684
- published: 06 Oct 2012
- views: 13684
Dennis Lee - Free Energy Demonstration - Unconvincing
- Order: Reorder
- Duration: 3:23:05
- Updated: 06 Oct 2015
- views: 4195
- published: 06 Oct 2015
- views: 4195
Free Energy, the embarrassing Truth
- Order: Reorder
- Duration: 33:23
- Updated: 23 Dec 2014
- views: 27959
- published: 23 Dec 2014
- views: 27959
The Searl Effect, Free Energy Generator - Documentary
- Order: Reorder
- Duration: 28:25
- Updated: 29 May 2017
- views: 7875
- published: 29 May 2017
- views: 7875
Introduction to Free-Energy Calculations - Chris Chipot
- Order: Reorder
- Duration: 1:31:10
- Updated: 14 Oct 2015
- views: 1434
- published: 14 Oct 2015
- views: 1434
Free Energy pt1
- Order: Reorder
- Duration: 24:43
- Updated: 06 Apr 2017
- views: 1577


- Playlist
- Chat

The Laws of Thermodynamics, Entropy, and Gibbs Free Energy
- Report rights infringement
- published: 16 Dec 2015
- views: 68236

Gibbs Free Energy - Equilibrium Constant, Enthalpy & Entropy - Equations & Practice Problems
- Report rights infringement
- published: 18 Jul 2016
- views: 1989

Free Energy Light Bulbs
- Report rights infringement
- published: 13 Jun 2017
- views: 5271
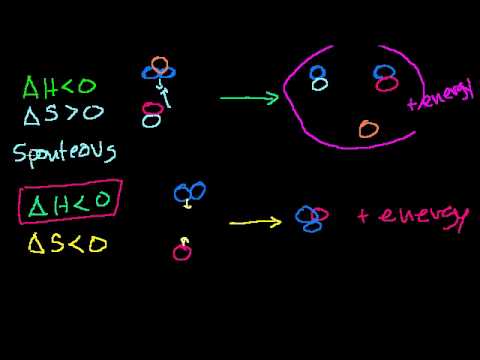
Gibbs free energy and spontaneity | Chemistry | Khan Academy
- Report rights infringement
- published: 28 Sep 2009
- views: 313264

Using Gibbs Free Energy
- Report rights infringement
- published: 14 Jan 2014
- views: 141989

Entropy: Embrace the Chaos! Crash Course Chemistry #20
- Report rights infringement
- published: 02 Jul 2013
- views: 645804

Gibbs Free Energy
- Report rights infringement
- published: 30 Jun 2011
- views: 385779

Thermodynamic Free Energy
- Report rights infringement
- published: 02 Nov 2011
- views: 5161

Ac Generator 220 volt 100 Watt free energy Part 2 with strong proof.... ( Et Discover)
- Report rights infringement
- published: 22 Jun 2017
- views: 191

Second Law of Thermodynamics,Entropy &Gibbs; Free Energy
- Report rights infringement
- published: 09 Dec 2012
- views: 128609

- Playlist
- Chat

The Laws of Thermodynamics, Entropy, and Gibbs Free Energy
- Report rights infringement
- published: 16 Dec 2015
- views: 68236

Gibbs Free Energy - Equilibrium Constant, Enthalpy & Entropy - Equations & Practice Problems
- Report rights infringement
- published: 18 Jul 2016
- views: 1989

Free Energy Light Bulbs
- Report rights infringement
- published: 13 Jun 2017
- views: 5271
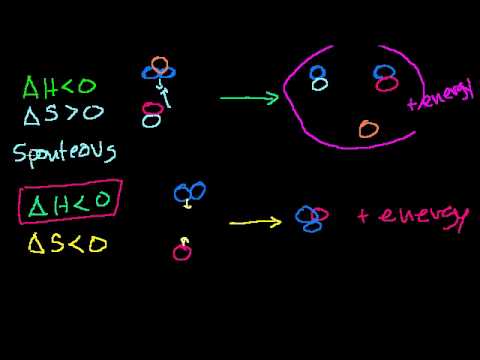
Gibbs free energy and spontaneity | Chemistry | Khan Academy
- Report rights infringement
- published: 28 Sep 2009
- views: 313264

Using Gibbs Free Energy
- Report rights infringement
- published: 14 Jan 2014
- views: 141989

Entropy: Embrace the Chaos! Crash Course Chemistry #20
- Report rights infringement
- published: 02 Jul 2013
- views: 645804

Gibbs Free Energy
- Report rights infringement
- published: 30 Jun 2011
- views: 385779

Thermodynamic Free Energy
- Report rights infringement
- published: 02 Nov 2011
- views: 5161

Ac Generator 220 volt 100 Watt free energy Part 2 with strong proof.... ( Et Discover)
- Report rights infringement
- published: 22 Jun 2017
- views: 191

Second Law of Thermodynamics,Entropy &Gibbs; Free Energy
- Report rights infringement
- published: 09 Dec 2012
- views: 128609

- Playlist
- Chat

Gibbs Free Energy - Equilibrium Constant, Enthalpy & Entropy - Equations & Practice Problems
- Report rights infringement
- published: 18 Jul 2016
- views: 1989

Thermodynamics 37 : Gibbs Helmholtz Free Energies
- Report rights infringement
- published: 01 Sep 2013
- views: 13757

Thermodynamics-Gibbs Free Energy
- Report rights infringement
- published: 31 Mar 2016
- views: 456

Thermodynamics, Entropy, Enthalpy & Gibbs Free Energy
- Report rights infringement
- published: 21 Dec 2013
- views: 20990

03 CBSE Class XI Chemistry Thermodynamics Entropy and Gibbs Energy
- Report rights infringement
- published: 06 Oct 2012
- views: 13684

Dennis Lee - Free Energy Demonstration - Unconvincing
- Report rights infringement
- published: 06 Oct 2015
- views: 4195

Free Energy, the embarrassing Truth
- Report rights infringement
- published: 23 Dec 2014
- views: 27959

The Searl Effect, Free Energy Generator - Documentary
- Report rights infringement
- published: 29 May 2017
- views: 7875

Introduction to Free-Energy Calculations - Chris Chipot
- Report rights infringement
- published: 14 Oct 2015
- views: 1434

Report: Human Rights Group 'Confirms' ISIS Leader Baghdadi Has Been Killed
Edit WorldNews.com 11 Jul 2017New Details Emerge On Trump Tower Meeting Between Donald Trump Jr. And Russian Lawyer
Edit WorldNews.com 11 Jul 2017Electric car maker scraps plans for $1 billion Nevada plant
Edit The Tribune San Luis Obispo 11 Jul 2017Dozens Form Human Chain To Rescue Family Swept Away By Riptide in Florida
Edit WorldNews.com 11 Jul 2017China says 'China responsibility theory' on North Korea has to stop
Edit DNA India 11 Jul 2017Spontaneous system follows rules of equilibrium
Edit PhysOrg 11 Jul 2017Microscopic dynamics of charge separation at the aqueous electrochemical interface [Chemistry]
Edit PNAS 11 Jul 2017Advanced Phase Change Material Market Foraying into Emerging Economies by 2020
Edit Community news 11 Jul 2017Mike Thompson: The Donald Trump Jr. defense
Edit Detroit Free Press 11 Jul 2017WA Portland Oregon Zone Forecast
Edit San Francisco Chronicle 11 Jul 2017Free Slurpees On “7-Eleven Day”
Edit CBS Sports 11 Jul 2017Free Slurpee Day At 7-Eleven Today: ‘Fox & Friends’ Talk Slurpee Turning Breech Baby Hearsay
Edit Inquisitr 11 Jul 2017Here’s how you can get free 7-Eleven Slurpees on July 11
Edit Atlanta Journal 11 Jul 2017Vatican bans use of gluten-free holy bread during Mass
Edit Irish Independent 11 Jul 2017Freebie Alert: 7-Eleven Has Free Slurpees Today
Edit Time Magazine 11 Jul 2017Here's how you can get free 7-Eleven Slurpees on July 11
Edit WPXI 11 Jul 2017Get Free Food At Chick-Fil-A By Dressing Up Like A Cow On July 11: Get ...
Edit Inquisitr 11 Jul 2017Solar eclipse 2017: Libraries across the U.S. are handing out free eclipse glasses
Edit Syracuse 11 Jul 2017Ministers mull ‘scaling back’ free schools plan to plug education budget gap
Edit Belfast Telegraph 11 Jul 2017- 1
- 2
- 3
- 4
- 5
- Next page »




























