- published: 27 Apr 2010
- views: 49365
-
remove the playlistAmorphous Solid
- remove the playlistAmorphous Solid
- published: 07 Oct 2013
- views: 400794
- published: 07 Jan 2014
- views: 7239
- published: 04 Jul 2015
- views: 102894
- published: 04 Dec 2012
- views: 18138
- published: 26 Sep 2016
- views: 1955
- published: 30 Apr 2010
- views: 27732
- published: 19 Dec 2014
- views: 15132

Amorphous solid
In condensed matter physics and materials science, an amorphous (from the Greek a, without, morphé, shape, form) or non-crystalline solid is a solid that lacks the long-range order characteristic of a crystal. In some older books, the term has been used synonymously with glass. Nowadays, "amorphous solid" is considered to be the overarching concept, and glass the more special case: A glass is an amorphous solid that exhibits a glass transition. Polymers are often amorphous. Other types of amorphous solids include gels, thin films, and nanostructured materials such as glass.
Amorphous materials have an internal structure made of interconnected structural blocks. Whether a material is liquid or solid depends primarily on the connectivity between its elementary building blocks so that solids are characterized by a high degree of connectivity whereas structural blocks in fluids have lower connectivity (see figure on amorphous material states).
Nano-structured materials
Even amorphous materials have some shortrange order at the atomic length scale due to the nature of chemical bonding (see structure of liquids and glasses for more information on non-crystalline material structure). Furthermore, in very small crystals a large fraction of the atoms are the crystal; relaxation of the surface and interfacial effects distort the atomic positions, decreasing the structural order. Even the most advanced structural characterization techniques, such as x-ray diffraction and transmission electron microscopy, have difficulty in distinguishing between amorphous and crystalline structures on these length scales.
This article is licensed under the Creative Commons Attribution-ShareAlike 3.0 Unported License, which means that you can copy and modify it as long as the entire work (including additions) remains under this license.

Crash Course
Crash Course (also known as Driving Academy) is a 1988 made for television teen film directed by Oz Scott.
Plot
Crash Course centers on a group of high schoolers in a driver’s education class; many for the second or third time. The recently divorced teacher, super-passive Larry Pearl, is on thin ice with the football fanatic principal, Principal Paulson, who is being pressured by the district superintendent to raise driver’s education completion rates or lose his coveted football program. With this in mind, Principal Paulson and his assistant, with a secret desire for his job, Abner Frasier, hire an outside driver’s education instructor with a very tough reputation, Edna Savage, aka E.W. Savage, who quickly takes control of the class.
The plot focuses mostly on the students and their interactions with their teachers and each other. In the beginning, Rico is the loner with just a few friends, Chadley is the bookish nerd with few friends who longs to be cool and also longs to be a part of Vanessa’s life who is the young, friendly and attractive girl who had to fake her mother’s signature on her driver’s education permission slip. Kichi is the hip-hop Asian kid who often raps what he has to say and constantly flirts with Maria, the rich foreign girl who thinks that the right-of-way on the roadways always goes to (insert awesomely fake foreign Latino accent) “my father’s limo”. Finally you have stereotypical football meathead J.J., who needs to pass his English exam to keep his eligibility and constantly asks out and gets rejected by Alice, the tomboy whose father owns “Santini & Son” Concrete Company. Alice is portrayed as being the “son” her father wanted.
This article is licensed under the Creative Commons Attribution-ShareAlike 3.0 Unported License, which means that you can copy and modify it as long as the entire work (including additions) remains under this license.
- Loading...

-
 2:03
2:03Crystalline And Amorphous Solids
Crystalline And Amorphous SolidsCrystalline And Amorphous Solids
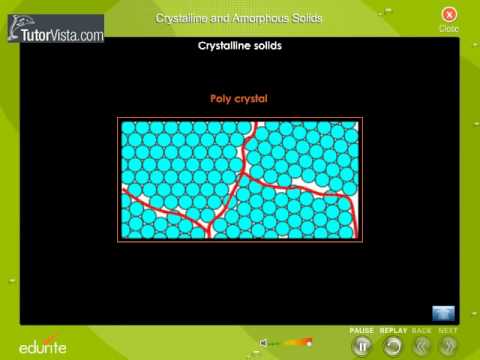
Follow us at: https://plus.google.com/+tutorvista/ Check us out at http://www.tutorvista.com/content/physics/physics-iii/solids-and-fluids/amorphous-solids.php Crystalline And Amorphous Solids Amorphous solids and crystalline solids if the size of the crystals is very small. Even amorphous materials have some short-range order at the atomic length scale due the nature of chemical bonding. Furthermore, in very small crystals a large fraction of the atoms are located at or near the surface of the crystal; relaxation of the surface and interfacial effects distort the atomic positions, decreasing the structural order. Even the most advanced structural characterization techniques, such as x-ray diffraction and transmission electron microscopy, have difficulty in distinguishing between amorphous and crystalline structures on these length scales.The transition from the liquid state to the glass, at a temperature below the equilibrium melting point of the material, is called the glass transition. The glass transition temperature, Tg, is the temperature at which an amorphous solid, such as glass or a polymer, becomes brittle on cooling, or soft on heating. More specifically, it defines a pseudo second order phase transition in which a supercooled melt yields, on cooling, a glassy structure and properties similar to those of crystalline materials e.g. of an isotropic solid material. Tg is usually applicable to wholly or partially amorphous solids such as common glasses and plastics (organic polymers). Below the glass transition temperature, Tg, amorphous solids are in a glassy state and most of their joining bonds are intact. In inorganic glasses, with increased temperature more and more joining bonds are broken by thermal fluctuations so that broken bonds (termed configurons) begin to form clusters. Above Tg these clusters become macroscopic large facilitating the flow of material. In organic polymers, secondary, non-covalent bonds between the polymer chains become weak above Tg. Above Tg glasses and organic polymers become soft and capable of plastic deformation without fracture. This behavior is one of the things which make most plastics useful . It is important to note that the glass transition temperature is a kinetic parameter, and thus parametrically depends on the melt cooling rate. Thus the slower the melt cooling rate, the lower Tg. In addition, Tg depends on the measurement conditions, which are not universally defined . The glass transition temperature is approximately the temperature at which the viscosity of the liquid exceeds a certain value (about 1012 Pa•s). The transition temperature depends on cooling rate, with the glass transition occurring at higher temperatures for faster cooling rates. The precise nature of the glass transition is the subject of ongoing research. While it is clear that the glass transition is not a first-order thermodynamic transition (such as melting), there is debate as to whether it is a higher-order transition such percolation type transformation , or merely a kinetic effect. The process of forming a crystalline structure from a fluid or from materials dissolved in the fluid is often referred to as crystallization. In the old example referenced by the root meaning of the word crystal, water being cooled undergoes a phase change from liquid to solid beginning with small ice crystals that grow until they fuse, forming a polycrystalline structure. The physical properties of the ice depend on the size and arrangement of the individual crystals, or grains, and the same may be said of metals solidifying from a molten state.Which crystal structure the fluid will form depends on the chemistry of the fluid, the conditions under which it is being solidified, and also on the ambient pressure. While the cooling process usually results in the generation of a crystalline material, under certain conditions, the fluid may be frozen in a noncrystalline state. In most cases, this involves cooling the fluid so rapidly that atoms cannot travel to their lattice sites before they lose mobility. A noncrystalline material, which has no long-range order, is called an amorphous, vitreous, or glassy material. Please like our facebook page http://www.facebook.com/tutorvista -
 9:18
9:18Doing Solids: Crash Course Chemistry #33
Doing Solids: Crash Course Chemistry #33Doing Solids: Crash Course Chemistry #33
You can directly support Crash Course at http://www.subbable.com/crashcourse Subscribe for as little as $0 to keep up with everything we're doing. Also, if you can afford to pay a little every month, it really helps us to continue producing great content. In which Hank blows our minds with the different kinds of Solids out there and talks about why they're all different and have different properties. Today, you'll learn about amorphous and crystalline solids, types of crystalline solids, types of crystalline atomic solids, properties of each type of solid, and that the properties depend on the bond types. ***** AND NOW, A SUBBABLE MESSAGE! ***** "Don't forget to be awesome, Dad! (For Tony) (Benji assisted)" - Daniel Smith -- Table of Contents Amorphous and Crystalline Solids 1:27 Types of Crystalline Solids 4:07 Types of Crystalline Atomic Solids 5:17 Properties of Each Type of Solid 4:16 Properties Depend on Bond Types 6:17 -- Want to find Crash Course elsewhere on the internet? Facebook - http://www.facebook.com/YouTubeCrashCourse Twitter - http://www.twitter.com/TheCrashCourse Tumblr - http://thecrashcourse.tumblr.com Support CrashCourse on Subbable: http://subbable.com/crashcourse -
 2:35
2:35Amorphous Solids
Amorphous SolidsAmorphous Solids
Learn about amorphous solids and their unique properties. Also get a better understanding of their rigid form. Make sure you are ready for test day. Visit: http://www.mometrix.com/academy/amorphous-solids/ Subscribe to more free test preparation videos: http://bit.ly/1dJH1yb Follow Mometrix Academy on Pinterest: http://bit.ly/1hZE2Jj Review our free test prep directory: http://bit.ly/1hZE2Jj Learn more About Us: http://bit.ly/1ewIADC #MometrixAcademy #AmorphousSolids -
 43:03
43:0313.1 Amorphous vs crystalline solids & classification of solids
13.1 Amorphous vs crystalline solids & classification of solids13.1 Amorphous vs crystalline solids & classification of solids
-
 0:55
0:55Amorphous Solid - Science Experiment
Amorphous Solid - Science ExperimentAmorphous Solid - Science Experiment
-
 3:22
3:22Is Glass Really A Solid?
Is Glass Really A Solid?Is Glass Really A Solid?
Of course glass is a solid… right? Could it possibly be a liquid? Read More: Physicists shatter stubborn mystery of how glass forms http://www.eurekalert.org/pub_releases/2015-06/uow-pss062915.php “A physicist at the University of Waterloo is among a team of scientists who have described how glasses form at the molecular level and provided a possible solution to a problem that has stumped scientists for decades.” Fact or Fiction?: Glass Is a (Supercooled) Liquid http://www.scientificamerican.com/article/fact-fiction-glass-liquid/ “Glass, however, is actually neither a liquid—supercooled or otherwise—nor a solid. It is an amorphous solid—a state somewhere between those two states of matter. “ What makes glass transparent? http://science.howstuffworks.com/question4041.htm ____________________ DNews is dedicated to satisfying your curiosity and to bringing you mind-bending stories & perspectives you won't find anywhere else! New videos twice daily. Watch More DNews on TestTube http://testtube.com/dnews Subscribe now! http://www.youtube.com/subscription_center?add_user=dnewschannel DNews on Twitter http://twitter.com/dnews Trace Dominguez on Twitter https://twitter.com/tracedominguez Julia Wilde on Twitter https://twitter.com/julia_sci DNews on Facebook https://facebook.com/DiscoveryNews DNews on Google+ http://gplus.to/dnews Discovery News http://discoverynews.com Download the TestTube App: http://testu.be/1ndmmMq -
 5:10
5:10Crystalline Solids and Amorphous Solids
Crystalline Solids and Amorphous SolidsCrystalline Solids and Amorphous Solids
Donate here: http://www.aklectures.com/donate.php Website video link: http://www.aklectures.com/lecture/crystalline-solids-and-amorphous-solids Facebook link: https://www.facebook.com/aklectures Website link: http://www.aklectures.com -
 16:31
16:31Classification of Solids - Crystalline and Amorphous solids
Classification of Solids - Crystalline and Amorphous solidsClassification of Solids - Crystalline and Amorphous solids
Tutorial for classification of solids including amorphous solids, crystalline solids and their properties by chemistryconcept -
 1:56
1:56Crystalline And Amorphous Solids
Crystalline And Amorphous SolidsCrystalline And Amorphous Solids
Check us out at http://www.tutorvista.com/content/physics/physics-iii/solids-and-fluids/amorphous-solids.php Crystalline and Amorphous Solids The process of forming a crystalline structure from a fluid or from materials dissolved in the fluid is often referred to as crystallization. In the old example referenced by the root meaning of the word crystal, water being cooled undergoes a phase change from liquid to solid beginning with small ice crystals that grow until they fuse, forming a polycrystalline structure. The physical properties of the ice depend on the size and arrangement of the individual crystals, or grains, and the same may be said of metals solidifying from a molten state. Which crystal structure the fluid will form depends on the chemistry of the fluid, the conditions under which it is being solidified, and also on the ambient pressure. While the cooling process usually results in the generation of a crystalline material, under certain conditions, the fluid may be frozen in a noncrystalline state. In most cases, this involves cooling the fluid so rapidly that atoms cannot travel to their lattice sites before they lose mobility. A noncrystalline material, which has no long-range order, is called an amorphous, vitreous, or glassy material. It is also often referred to as an amorphous solid, although there are distinct differences between crystalline solids and amorphous solids: most notably, the process of forming a glass does not release the latent heat of fusion. Crystalline structures occur in all classes of materials, with all types of chemical bonds. Almost all metal exists in a polycrystalline state; amorphous or single-crystal metals must be produced synthetically, often with great difficulty. Ionically bonded crystals can form upon solidification of salts, either from a molten fluid or upon crystallization from a solution. Covalently bonded crystals are also very common, notable examples being diamond, silica, and graphite. Polymer materials generally will form crystalline regions, but the lengths of the molecules usually prevent complete crystallization. Weak van der Waals forces can also play a role in a crystal structure; for example, this type of bonding loosely holds together the hexagonal-patterned sheets in graphite. Most crystalline materials have a variety of crystallographic defects. The types and structures of these defects can contain a profound effect on the properties of the materials. An "amorphous solid" is a solid in which there is no long-range order of the positions of the atoms. (Solids in which there is long-range atomic order are called crystallines or morphous). Most classes of solid materials can be found or prepared in an amorphous form. For instance, common window glass is an amorphous solid, many polymers (such as polystyrene) are amorphous, and even foods such as cotton candy are amorphous solids. In principle, given a sufficiently high cooling rate, any liquid can be made into an amorphous solid. Cooling reduces molecular mobility. If the cooling rate is faster than the rate at which molecules can organize into a more thermodynamically favorable crystalline state, then an amorphous solid will be formed. Because of entropy considerations, many polymers can be made amorphous solids by cooling even at slow rates. In contrast, if molecules have sufficient time to organize into a structure with two- or three-dimensional order, then a crystalline (or semi-crystalline) solid will be formed. Water is one example. Because of its small molecular size and ability to quickly rearrange, it cannot be made amorphous without resorting to specialized hyperquenching techniques. Amorphous materials can also be produced by additives which interfere with the ability of the primary constituent to crystallize. For example, addition of soda to silicon dioxide results in window glass, and the addition of glycols to water results in a vitrified solid. Some materials, such as metals, are difficult to prepare in an amorphous state. Unless a material has a high melting temperature (as ceramics do) or a low crystallization energy (as polymers tend to), cooling must be done extremely rapidly. As the cooling is performed, the material changes from a supercooled liquid, with properties one would expect from a liquid state material, to a solid. The temperature at which this transition occurs is called the glass transition temperature or Tg. Please like our facebook page http://www.facebook.com/tutorvista Follow us at: https://plus.google.com/+tutorvista/ -
 13:49
13:49Single Crystal, Polycrystalline, Amorphous {Texas A&M;: Intro to Materials}
Single Crystal, Polycrystalline, Amorphous {Texas A&M;: Intro to Materials}Single Crystal, Polycrystalline, Amorphous {Texas A&M;: Intro to Materials}
Tutorial covering the basics of atomic and microstructure and relating material form back to material properties (isotropic/anisotropic). Video lecture for Introduction to Materials Science & Engineering (MSEN 201/MEEN 222), Texas A&M; University, College Station, TX. http://engineering.tamu.edu/materials
-

Crystalline And Amorphous Solids
Follow us at: https://plus.google.com/+tutorvista/ Check us out at http://www.tutorvista.com/content/physics/physics-iii/solids-and-fluids/amorphous-solids.php Crystalline And Amorphous Solids Amorphous solids and crystalline solids if the size of the crystals is very small. Even amorphous materials have some short-range order at the atomic length scale due the nature of chemical bonding. Furthermore, in very small crystals a large fraction of the atoms are located at or near the surface of the crystal; relaxation of the surface and interfacial effects distort the atomic positions, decreasing the structural order. Even the most advanced structural characterization techniques, such as x-ray diffraction and transmission electron microscopy, have difficulty in distinguishing between amorp...
published: 27 Apr 2010 -

Doing Solids: Crash Course Chemistry #33
You can directly support Crash Course at http://www.subbable.com/crashcourse Subscribe for as little as $0 to keep up with everything we're doing. Also, if you can afford to pay a little every month, it really helps us to continue producing great content. In which Hank blows our minds with the different kinds of Solids out there and talks about why they're all different and have different properties. Today, you'll learn about amorphous and crystalline solids, types of crystalline solids, types of crystalline atomic solids, properties of each type of solid, and that the properties depend on the bond types. ***** AND NOW, A SUBBABLE MESSAGE! ***** "Don't forget to be awesome, Dad! (For Tony) (Benji assisted)" - Daniel Smith -- Table of Contents Amorphous and Crystalline Solids 1:27 Typ...
published: 07 Oct 2013 -

Amorphous Solids
Learn about amorphous solids and their unique properties. Also get a better understanding of their rigid form. Make sure you are ready for test day. Visit: http://www.mometrix.com/academy/amorphous-solids/ Subscribe to more free test preparation videos: http://bit.ly/1dJH1yb Follow Mometrix Academy on Pinterest: http://bit.ly/1hZE2Jj Review our free test prep directory: http://bit.ly/1hZE2Jj Learn more About Us: http://bit.ly/1ewIADC #MometrixAcademy #AmorphousSolids
published: 07 Jan 2014 -

13.1 Amorphous vs crystalline solids & classification of solids
published: 04 Dec 2013 -

Amorphous Solid - Science Experiment
published: 14 Oct 2013 -

Is Glass Really A Solid?
Of course glass is a solid… right? Could it possibly be a liquid? Read More: Physicists shatter stubborn mystery of how glass forms http://www.eurekalert.org/pub_releases/2015-06/uow-pss062915.php “A physicist at the University of Waterloo is among a team of scientists who have described how glasses form at the molecular level and provided a possible solution to a problem that has stumped scientists for decades.” Fact or Fiction?: Glass Is a (Supercooled) Liquid http://www.scientificamerican.com/article/fact-fiction-glass-liquid/ “Glass, however, is actually neither a liquid—supercooled or otherwise—nor a solid. It is an amorphous solid—a state somewhere between those two states of matter. “ What makes glass transparent? http://science.howstuffworks.com/question4041.htm ___...
published: 04 Jul 2015 -

Crystalline Solids and Amorphous Solids
Donate here: http://www.aklectures.com/donate.php Website video link: http://www.aklectures.com/lecture/crystalline-solids-and-amorphous-solids Facebook link: https://www.facebook.com/aklectures Website link: http://www.aklectures.com
published: 04 Dec 2012 -

Classification of Solids - Crystalline and Amorphous solids
Tutorial for classification of solids including amorphous solids, crystalline solids and their properties by chemistryconcept
published: 26 Sep 2016 -

Crystalline And Amorphous Solids
Check us out at http://www.tutorvista.com/content/physics/physics-iii/solids-and-fluids/amorphous-solids.php Crystalline and Amorphous Solids The process of forming a crystalline structure from a fluid or from materials dissolved in the fluid is often referred to as crystallization. In the old example referenced by the root meaning of the word crystal, water being cooled undergoes a phase change from liquid to solid beginning with small ice crystals that grow until they fuse, forming a polycrystalline structure. The physical properties of the ice depend on the size and arrangement of the individual crystals, or grains, and the same may be said of metals solidifying from a molten state. Which crystal structure the fluid will form depends on the chemistry of the fluid, the conditions under...
published: 30 Apr 2010 -

Single Crystal, Polycrystalline, Amorphous {Texas A&M;: Intro to Materials}
Tutorial covering the basics of atomic and microstructure and relating material form back to material properties (isotropic/anisotropic). Video lecture for Introduction to Materials Science & Engineering (MSEN 201/MEEN 222), Texas A&M; University, College Station, TX. http://engineering.tamu.edu/materials
published: 19 Dec 2014
Crystalline And Amorphous Solids
- Order: Reorder
- Duration: 2:03
- Updated: 27 Apr 2010
- views: 49365
- published: 27 Apr 2010
- views: 49365
Doing Solids: Crash Course Chemistry #33
- Order: Reorder
- Duration: 9:18
- Updated: 07 Oct 2013
- views: 400794
- published: 07 Oct 2013
- views: 400794
Amorphous Solids
- Order: Reorder
- Duration: 2:35
- Updated: 07 Jan 2014
- views: 7239
- published: 07 Jan 2014
- views: 7239
13.1 Amorphous vs crystalline solids & classification of solids
- Order: Reorder
- Duration: 43:03
- Updated: 04 Dec 2013
- views: 11733
- published: 04 Dec 2013
- views: 11733
Amorphous Solid - Science Experiment
- Order: Reorder
- Duration: 0:55
- Updated: 14 Oct 2013
- views: 562
- published: 14 Oct 2013
- views: 562
Is Glass Really A Solid?
- Order: Reorder
- Duration: 3:22
- Updated: 04 Jul 2015
- views: 102894
- published: 04 Jul 2015
- views: 102894
Crystalline Solids and Amorphous Solids
- Order: Reorder
- Duration: 5:10
- Updated: 04 Dec 2012
- views: 18138
- published: 04 Dec 2012
- views: 18138
Classification of Solids - Crystalline and Amorphous solids
- Order: Reorder
- Duration: 16:31
- Updated: 26 Sep 2016
- views: 1955
- published: 26 Sep 2016
- views: 1955
Crystalline And Amorphous Solids
- Order: Reorder
- Duration: 1:56
- Updated: 30 Apr 2010
- views: 27732
- published: 30 Apr 2010
- views: 27732
Single Crystal, Polycrystalline, Amorphous {Texas A&M;: Intro to Materials}
- Order: Reorder
- Duration: 13:49
- Updated: 19 Dec 2014
- views: 15132
- published: 19 Dec 2014
- views: 15132

- Playlist
- Chat

- Playlist
- Chat

Crystalline And Amorphous Solids
- Report rights infringement
- published: 27 Apr 2010
- views: 49365

Doing Solids: Crash Course Chemistry #33
- Report rights infringement
- published: 07 Oct 2013
- views: 400794

Amorphous Solids
- Report rights infringement
- published: 07 Jan 2014
- views: 7239

13.1 Amorphous vs crystalline solids & classification of solids
- Report rights infringement
- published: 04 Dec 2013
- views: 11733

Amorphous Solid - Science Experiment
- Report rights infringement
- published: 14 Oct 2013
- views: 562

Is Glass Really A Solid?
- Report rights infringement
- published: 04 Jul 2015
- views: 102894

Crystalline Solids and Amorphous Solids
- Report rights infringement
- published: 04 Dec 2012
- views: 18138

Classification of Solids - Crystalline and Amorphous solids
- Report rights infringement
- published: 26 Sep 2016
- views: 1955

Crystalline And Amorphous Solids
- Report rights infringement
- published: 30 Apr 2010
- views: 27732

Single Crystal, Polycrystalline, Amorphous {Texas A&M;: Intro to Materials}
- Report rights infringement
- published: 19 Dec 2014
- views: 15132
Pop superstar Beyonce gives birth to twins, hints her dad
Edit The Jakarta Post 19 Jun 2017'Donald Trump's Cuba policy takes us back to the Cold War era'
Edit Mid Day 19 Jun 2017US Shoots Down Syria Warplane in Raqqa Province
Edit News18 19 Jun 2017Marine Le Pen elected French MP but her far-right FN party faces debacle
Edit Hindustan Times 19 Jun 2017Philippine government prepares ceasefire with rebels to focus on fight against militants in Marawi
Edit South China Morning Post 19 Jun 2017From suffering insolence by Jesus Christ to not even meriting a villainous character in popular ...
Edit The Daily Beast 18 Jun 2017Britons have got a right to 'reel' in the face of terror attacks
Edit The Observer 18 Jun 2017Oklahoma artists Romy Owens, Adam Lanman reach for stars on Enid public art project
Edit The Oklahoman 18 Jun 2017Oklahoma artists Romy Owens and Adam Lanman reach for the stars on their Enid public art project 'Under Her Wing Was the Universe'
Edit The Oklahoman 18 Jun 2017It was a probe without evidence, says Justice BN Srikrishna
Edit DNA India 17 Jun 2017Probe in 1993 Mumbai Blasts Conducted Without Evidence: Former SC Judge
Edit Yahoo Daily News 17 Jun 2017From Pseudo-Democracy to Real Participation
Edit CounterPunch 16 Jun 2017Stu Bykofsky: Calls for gun limits predictable, unrealistic
Edit The Spokesman-Review 15 Jun 2017Solar Photovoltaic Material Market - Global Industry Analysis 2024 | TMR
Edit Community news 15 Jun 2017High Performance Plastics Market -Trends, Outlook, Global Industry Insights, and Opportunity Analysis, 2016–2024
Edit Community news 15 Jun 2017- 1
- 2
- 3
- 4
- 5
- Next page »
















