- published: 12 Mar 2013
- views: 1310538
-
remove the playlistElectrons
- remove the playlistElectrons
- published: 19 Nov 2009
- views: 566544
- published: 26 Feb 2008
- views: 445044
- published: 19 Dec 2012
- views: 451317
- published: 21 Dec 2012
- views: 150307
- published: 03 Dec 2015
- views: 15606
- published: 04 Aug 2013
- views: 817637
- published: 28 Nov 2012
- views: 220180
- published: 28 Oct 2011
- views: 48940
- published: 07 May 2014
- views: 13241

Electron
The electron is a subatomic particle, symbol e− or β−, with a negative elementary electric charge. Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure. The electron has a mass that is approximately 1/1836 that of the proton.Quantum mechanical properties of the electron include an intrinsic angular momentum (spin) of a half-integer value in units of ħ, which means that it is a fermion. Being fermions, no two electrons can occupy the same quantum state, in accordance with the Pauli exclusion principle. Like all matter, electrons have properties of both particles and waves, and so can collide with other particles and can be diffracted like light. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a higher De Broglie wavelength for typical energies.
This article is licensed under the Creative Commons Attribution-ShareAlike 3.0 Unported License, which means that you can copy and modify it as long as the entire work (including additions) remains under this license.

Wikipedia
Wikipedia (![]() i/ˌwɪkᵻˈpiːdiə/ or
i/ˌwɪkᵻˈpiːdiə/ or ![]() i/ˌwɪkiˈpiːdiə/ WIK-i-PEE-dee-ə) is a free-access, free-content Internet encyclopedia, supported and hosted by the non-profit Wikimedia Foundation. Those who can access the site can edit most of its articles. Wikipedia is ranked among the ten most popular websites, and constitutes the Internet's largest and most popular general reference work.
i/ˌwɪkiˈpiːdiə/ WIK-i-PEE-dee-ə) is a free-access, free-content Internet encyclopedia, supported and hosted by the non-profit Wikimedia Foundation. Those who can access the site can edit most of its articles. Wikipedia is ranked among the ten most popular websites, and constitutes the Internet's largest and most popular general reference work.
Jimmy Wales and Larry Sanger launched Wikipedia on January 15, 2001. Sanger coined its name, a portmanteau of wiki and encyclopedia. Initially only in English, Wikipedia quickly became multilingual as it developed similar versions in other languages, which differ in content and in editing practices. The English Wikipedia is now one of 291 Wikipedia editions and is the largest with 5,081,662 articles (having reached 5,000,000 articles in November 2015). There is a grand total, including all Wikipedias, of over 38 million articles in over 250 different languages. As of February 2014, it had 18 billion page views and nearly 500 million unique visitors each month.
This article is licensed under the Creative Commons Attribution-ShareAlike 3.0 Unported License, which means that you can copy and modify it as long as the entire work (including additions) remains under this license.

2013
2013 (MMXIII) was a common year starting on Tuesday (dominical letter F) of the Gregorian calendar, the 2013th year of the Common Era (CE) and Anno Domini (AD) designations, the 13th year of the 3rd millennium, the 13th year of the 21st century, and the 4th year of the 2010s decade.
2013 was designated as:
Events
January
February
This article is licensed under the Creative Commons Attribution-ShareAlike 3.0 Unported License, which means that you can copy and modify it as long as the entire work (including additions) remains under this license.

Standard Model
The Standard Model of particle physics is a theory concerning the electromagnetic, weak, and strong nuclear interactions, as well as classifying all the subatomic particles known. It was developed throughout the latter half of the 20th century, as a collaborative effort of scientists around the world. The current formulation was finalized in the mid-1970s upon experimental confirmation of the existence of quarks. Since then, discoveries of the top quark (1995), the tau neutrino (2000), and more recently the Higgs boson (2012), have given further credence to the Standard Model. Because of its success in explaining a wide variety of experimental results, the Standard Model is sometimes regarded as a "theory of almost everything".
Although the Standard Model is believed to be theoretically self-consistent and has demonstrated huge and continued successes in providing experimental predictions, it does leave some phenomena unexplained and it falls short of being a complete theory of fundamental interactions. It does not incorporate the full theory of gravitation as described by general relativity, or account for the accelerating expansion of the universe (as possibly described by dark energy). The model does not contain any viable dark matter particle that possesses all of the required properties deduced from observational cosmology. It also does not incorporate neutrino oscillations (and their non-zero masses).
This article is licensed under the Creative Commons Attribution-ShareAlike 3.0 Unported License, which means that you can copy and modify it as long as the entire work (including additions) remains under this license.
File
File or filing may refer to:
Services
Information
Computing
- file URI scheme
- file (command), a Unix program for determining the type of data contained in a computer file
Other uses
See also
This article is licensed under the Creative Commons Attribution-ShareAlike 3.0 Unported License, which means that you can copy and modify it as long as the entire work (including additions) remains under this license.
- Loading...

-
 12:48
12:48The Electron: Crash Course Chemistry #5
The Electron: Crash Course Chemistry #5The Electron: Crash Course Chemistry #5
Hank brings us the story of the electron and describes how reality is a kind of music, discussing electron shells and orbitals, electron configurations, ionization and electron affinities, and how all these things can be understood via the periodic table. Crash Course on the internet! http://www.facebook.com/YouTubeCrashCourse http://www.twitter.com/TheCrashCourse http://TheCrashCourse.tumblr.com Table of Contents Snobby Scientists 00:43 Great Dane/Bohr Model 01:57 Electrons as Music 04:13 Electron Shells and Orbitals 04:44 Electron Configurations 05:54 Ionization and Electron Affinities 08:17 Periodic Table 10:18 Support CrashCourse on Subbable: http://subbable.com/crashcourse -
 7:44
7:44Electrons, Protons And Neutrons | Standard Model Of Particle Physics
Electrons, Protons And Neutrons | Standard Model Of Particle PhysicsElectrons, Protons And Neutrons | Standard Model Of Particle Physics
http://www.facebook.com/ScienceReason ... The Standard Model of Particle Physics (Chapter 5): Electrons, Protons And Neutrons. --- Please SUBSCRIBE to Science & Reason: • http://www.youtube.com/Best0fScience • http://www.youtube.com/ScienceTV • http://www.youtube.com/FFreeThinker --- STANDARD MODEL OF PARTICLE PHYSICS: http://www.youtube.com/user/Best0fScience#g/c/4A8C50311C9F7369 1) First Second Of The Universe: http://www.youtube.com/watch?v=4HXPYO5YFG0 2) Force And Matter: http://www.youtube.com/watch?v=p5QXZ0__8VU 3) Quarks: http://www.youtube.com/watch?v=PxQwkdu9WbE 4) Gluons: http://www.youtube.com/watch?v=ZYPem05vpS4 5) Electrons, Protons And Neutrons: http://www.youtube.com/watch?v=Vi91qyjuknM 6) Photons, Gravitons & Weak Bosons: http://www.youtube.com/watch?v=JHVC6F8SOFc 7) Neutrinos: http://www.youtube.com/watch?v=m7QAaH0oFNg 8) The Higgs Boson / The Higgs Mechanism: http://www.youtube.com/watch?v=1_HrQVhgbeo The Standard Model of particle physics is a theory of three of the four known fundamental interactions and the elementary particles that take part in these interactions. These particles make up all visible matter in the universe. Every high energy physics experiment carried out since the mid-20th century has eventually yielded findings consistent with the Standard Model. Still, the Standard Model falls short of being a complete theory of fundamental interactions because it does not include gravitation, dark matter, or dark energy. It is not quite a complete description of leptons either, because it does not describe nonzero neutrino masses, although simple natural extensions do. • http://en.wikipedia.org/wiki/Standard_Model --- ELECTRONS The particle itself is a fundamental particle and is too small to be seen by any imaginable instrument of observation. So we instead represent the properties that allow the electron to interact. The central small dot represents the weak charge of the electron. This charge entirely separate from electric charge gives rise to the Weak Nuclear Force. This force causes radioactive decay and its typical range is much smaller than the diameter of a proton. The larger volume of shifting purple is meant to represent the Electric Charge of the electron. This charge is the generator of the Electromagnetic Force which has infinite range although the drop off in strength is pretty dramatic as we move away from the electron. The Electromagnetic Force is how electrons interact with other electrically charged particles and with magnetic fields. These interactions make the structure of atoms and molecules possible. This gives rise to almost all of the complexity that we see around us. PROTONS The Proton is composed of two up quarks and one down quark (as you can see from the tiny rings of color near the center of each quark.) The overall charge of the proton is positive and so we have given it a gold shell. (Note that we can simply add the charges of the individual quarks to get the charge of the proton). The red, green, and blue colors of the quarks represent the color charge which generates the Strong Nuclear Force that holds them together. It comes in three different charges represented here by the three colors, and for different colors the force is attractive. The mediator of the Strong Force (the particle that is exchanged in an interaction) is a gluon. We represent gluon exchange as the occasional wispy strings between the quarks. As you can see the gluons have color themselves, and each gluon exchange causes the quarks involved to swap color. Although we show the quark motion inside the proton as leisurely, they are actually traveling close to the speed of light. NEUTRONS The Neutron is composed of two down quarks and one up quark (as you can see from the tiny rings of color near the center of each quark.) The overall charge of the neutron is neutral and so we have given it a silver shell. (note that we can simply add the charges of the individual quarks to get the charge of the neutron. ) The red, green, and blue colors of the quarks represent the color charge that generates the Strong Nuclear Force that holds them together. It comes in three charges represented here by the three colors, and for different colors the force is attractive. The mediator of the Strong Force (the particle that is exchanged in an interaction) is a gluon. We represent gluon exchange as the occasional wispy strings between the quarks. As you can see the gluons have color themselves, and each gluon exchange causes the quarks involved to swap color. Although we show the quark motion inside the neutron as leisurely, they are actually traveling close to the speed of light. • http://www.cassiopeiaproject.com . -
 0:27
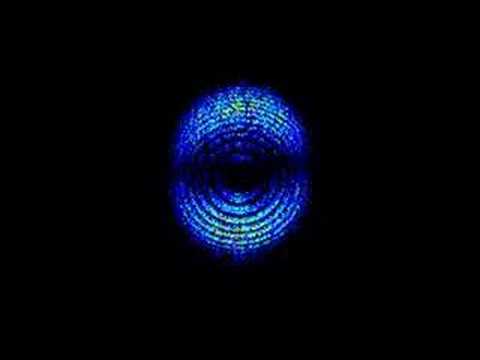
0:27Scientists in Sweden film moving electron for the first time
Scientists in Sweden film moving electron for the first timeScientists in Sweden film moving electron for the first time
Scientists in Sweden film the sub-atomic particle, the electron, for the first time. An electron is approximately 1867 times smaller than a proton and is constantly moving. -
 16:53
16:53Valence Electrons and the Periodic Table
Valence Electrons and the Periodic TableValence Electrons and the Periodic Table
To see all my Chemistry videos, check out http://socratic.org/chemistry Where do electrons live in atoms? They live in energy levels or shells, which are varying distances from the nucleus, and can hold varying numbers of electrons. The most important electrons in an atom are the valence electrons, which are in the outermost energy level or shell. We'll look at how to determine the number of valence electrons in an atom, based on which column it is in on the periodic table. -
 3:06
3:06How Fast is an Electron and Electricity
How Fast is an Electron and ElectricityHow Fast is an Electron and Electricity
How fast is an electron in a wire and how fast is electricity? An electron moves surprisingly slow, slower than a snail, while electricity moves at near the speed of light. Electrons move at what's called the drift velocity. This video illustrates all this in an entertaining and informative way. Enjoy! This video has correct English captions. Click on the CC button at the bottom of the video to see them. For "How Radiation Works - Americium 241, Alpha Particles and Gamma Rays", see: http://youtu.be/aJkx6hAD-4E For "How to make solar cells (DIY/homemade solar cell)", see: http://youtu.be/g5Edw99PgzQ For "Fresnel lens - what is it, testing focal length, solar heat generated", see: http://youtu.be/11n0ZaZMj3A To follow me on Twitter: https://twitter.com/#!/RimStarz http://rimstar.org 3D modelling and animation done using Blender 2.63. Original Earth image from NASA/courtesy of nasaimages.org. -
 34:26
34:26Atoms and Their Electrons
Atoms and Their ElectronsAtoms and Their Electrons
Eduction video from 1996 If you want to see more videos like this, click that like button and subscribe button!!! -
 10:17
10:17Electron Configuration
Electron ConfigurationElectron Configuration
005 - Electron Configuration In this video Paul Andersen explains how to write out the electron configuration for atoms on the periodic table. More importantly he shows you why electrons arrange themselves in shells, subshells and orbitals by using Coulomb's law and studying the first ionization energies of different atoms. ANSWERS: Cl - [Ne] 3s^2 3p^5 Ag - [Kr] 4d^10 5s^1 - Did you get [Kr] 5s^2 4d^9? There are a few exceptions to this law. Most of them are found in the f-block metals and they are not of much chemical significance. Music Attribution Title: String Theory Artist: Herman Jolly http://sunsetvalley.bandcamp.com/track/string-theory All of the images are licensed under creative commons and public domain licensing: File:Electron Configuration Diagrams from H to Ne.svg, n.d. http://commons.wikimedia.org/wiki/File:Electron_configuration_diagrams_from_H_to_Ne.svg. "File:Electron Orbitals.svg." Wikipedia, the Free Encyclopedia. Accessed July 31, 2013. https://en.wikipedia.org/wiki/File:Electron_orbitals.svg. "File:Electron Shell 001 Hydrogen - No Label.svg." Wikipedia, the Free Encyclopedia. Accessed July 31, 2013. http://en.wikipedia.org/wiki/File:Electron_shell_001_Hydrogen_-_no_label.svg. "File:Electron Shell 002 Helium - No Label.svg." Wikipedia, the Free Encyclopedia. Accessed July 31, 2013. http://en.wikipedia.org/wiki/File:Electron_shell_002_Helium_-_no_label.svg. "File:Electron Shell 003 Lithium - No Label.svg." Wikipedia, the Free Encyclopedia. Accessed July 31, 2013. http://en.wikipedia.org/wiki/File:Electron_shell_003_Lithium_-_no_label.svg. "File:Electron Shell 004 Beryllium - No Label.svg." Wikipedia, the Free Encyclopedia. Accessed July 31, 2013. http://en.wikipedia.org/wiki/File:Electron_shell_004_Beryllium_-_no_label.svg. "File:Empirical Atomic Radius Trends.png." Wikipedia, the Free Encyclopedia. Accessed August 1, 2013. http://en.wikipedia.org/wiki/File:Empirical_atomic_radius_trends.png. "File:First Ionization Energy.svg." Wikipedia, the Free Encyclopedia. Accessed August 1, 2013. http://en.wikipedia.org/wiki/File:First_Ionization_Energy.svg. "File:Klechkovski Rule.svg." Wikipedia, the Free Encyclopedia. Accessed July 31, 2013. http://en.wikipedia.org/wiki/File:Klechkovski_rule.svg. "File:Periodic Table 2.svg." Wikipedia, the Free Encyclopedia. Accessed July 31, 2013. https://en.wikipedia.org/wiki/File:Periodic_Table_2.svg. "File:Periodic Trends.svg." Wikipedia, the Free Encyclopedia. Accessed August 1, 2013. http://en.wikipedia.org/wiki/File:Periodic_trends.svg. "File:Periodic Variation of Pauling Electronegativities.png." Wikipedia, the Free Encyclopedia. Accessed August 1, 2013. http://en.wikipedia.org/wiki/File:Periodic_variation_of_Pauling_electronegativities.png. RJHall. Ionization Energies of Neutral Elements, in Units of eV., October 10, 2010. File:Ionization energies.png. http://commons.wikimedia.org/wiki/File:Ionization_energies.svg. -
 11:08
11:08Discovery of the Electron: Cathode Ray Tube Experiment
Discovery of the Electron: Cathode Ray Tube ExperimentDiscovery of the Electron: Cathode Ray Tube Experiment
To see all my Chemistry videos, check out http://socratic.org/chemistry J.J. Thompson discovered the electron, the first of the subatomic particles, using the cathode ray tube experiment. He found that many different metals release cathode rays, and that cathode rays were made of electrons, very small negatively charged particles. This disproved John Dalton's theory of the atom, and Thompson came up with the plum pudding model of the atom. -
 1:31
1:31How Electrons Become Electricity
How Electrons Become ElectricityHow Electrons Become Electricity
Electricity is the flow of electrons. This video shows how electrons move and become electricity using a power supply. This video was made as a part of the Quarked! website, a grant funded collaboration between the University of Kansas Department of Physics and Astronomy researchers and the educators at the Natural History Museum http://naturalhistory.ku.edu/. For more information, videos, and games, please visit http://www.quarked.org. © 2011 The University of Kansas -
 1:25
1:25The Science of Electrons - What is an Electron?
The Science of Electrons - What is an Electron?The Science of Electrons - What is an Electron?
Find me Around the Web: FACEBOOK - https://www.facebook.com/mrstargazer.nation TWITTER - https://twitter.com/STARGAZERNATION Blogspot - http://stargazernation.blogspot.co.uk/ On Pinterest: http://www.pinterest.com/Stargazernation/ Check out the latest: http://checkoutthelatest.com/MRSTARGAZERNATION Frequency: http://www.frequency.com/mrstargazernation?cid=4-2155340 Ashtar Command Crew: http://www.ashtarcommandcrew.net/profiles/blog/list?user=251glgsbn5l1o YouTube: https://www.youtube.com/user/MRSTARGAZERNATION Science Stage: http://sciencestage.com/stargazernation Before its News: http://beforeitsnews.com/contributor/pages/343/420/stories.html Blogster - http://www.blogster.com/stargazernation/
Electrons
ALBUMS
- Kid Kenobi Sessions 2007 released: 2007
Kid Kenobi Sessions 2007
Released 2007- add to main playlist Play in Full Screen The Drum (Groove Diggaz remix)
- add to main playlist Play in Full Screen Fancy Footwork (Guns & Bombs remix)
- add to main playlist Play in Full Screen Beer Chucker
- add to main playlist Play in Full Screen NYC Beat (MSTRKRFT remix)
- add to main playlist Play in Full Screen Ross Ross Ross
- add to main playlist Play in Full Screen Floating (Alex Metric remix)
- add to main playlist Play in Full Screen The Screetch
- add to main playlist Play in Full Screen Shake and Pop (Aston Shuffle remix)
- add to main playlist Play in Full Screen Organ Grinder
- add to main playlist Play in Full Screen Naughty (Kiss Sell Out remix)
- add to main playlist Play in Full Screen Make Me Sweat
- add to main playlist Play in Full Screen Off Da Hook!
- add to main playlist Play in Full Screen Get Up (General Electric version)
- add to main playlist Play in Full Screen Osc Low Nyzz (Blende remix)
- add to main playlist Play in Full Screen Love Is a Number (Kid Kenobi remix)
- add to main playlist Play in Full Screen Master System (Koma & Bones remix)
- add to main playlist Play in Full Screen We Are Your Friends (Mike 'Heavy Friends' remix)
- add to main playlist Play in Full Screen Banquet (Boys Noize remix)
-

The Electron: Crash Course Chemistry #5
Hank brings us the story of the electron and describes how reality is a kind of music, discussing electron shells and orbitals, electron configurations, ionization and electron affinities, and how all these things can be understood via the periodic table. Crash Course on the internet! http://www.facebook.com/YouTubeCrashCourse http://www.twitter.com/TheCrashCourse http://TheCrashCourse.tumblr.com Table of Contents Snobby Scientists 00:43 Great Dane/Bohr Model 01:57 Electrons as Music 04:13 Electron Shells and Orbitals 04:44 Electron Configurations 05:54 Ionization and Electron Affinities 08:17 Periodic Table 10:18 Support CrashCourse on Subbable: http://subbable.com/crashcourse
published: 12 Mar 2013 -

Electrons, Protons And Neutrons | Standard Model Of Particle Physics
http://www.facebook.com/ScienceReason ... The Standard Model of Particle Physics (Chapter 5): Electrons, Protons And Neutrons. --- Please SUBSCRIBE to Science & Reason: • http://www.youtube.com/Best0fScience • http://www.youtube.com/ScienceTV • http://www.youtube.com/FFreeThinker --- STANDARD MODEL OF PARTICLE PHYSICS: http://www.youtube.com/user/Best0fScience#g/c/4A8C50311C9F7369 1) First Second Of The Universe: http://www.youtube.com/watch?v=4HXPYO5YFG0 2) Force And Matter: http://www.youtube.com/watch?v=p5QXZ0__8VU 3) Quarks: http://www.youtube.com/watch?v=PxQwkdu9WbE 4) Gluons: http://www.youtube.com/watch?v=ZYPem05vpS4 5) Electrons, Protons And Neutrons: http://www.youtube.com/watch?v=Vi91qyjuknM 6) Photons, Gravitons & Weak Bosons: http://www.youtube.com/watch?v=JHVC6F8SOFc 7) Neu...
published: 19 Nov 2009 -

Scientists in Sweden film moving electron for the first time
Scientists in Sweden film the sub-atomic particle, the electron, for the first time. An electron is approximately 1867 times smaller than a proton and is constantly moving.
published: 26 Feb 2008 -

Valence Electrons and the Periodic Table
To see all my Chemistry videos, check out http://socratic.org/chemistry Where do electrons live in atoms? They live in energy levels or shells, which are varying distances from the nucleus, and can hold varying numbers of electrons. The most important electrons in an atom are the valence electrons, which are in the outermost energy level or shell. We'll look at how to determine the number of valence electrons in an atom, based on which column it is in on the periodic table.
published: 19 Dec 2012 -

How Fast is an Electron and Electricity
How fast is an electron in a wire and how fast is electricity? An electron moves surprisingly slow, slower than a snail, while electricity moves at near the speed of light. Electrons move at what's called the drift velocity. This video illustrates all this in an entertaining and informative way. Enjoy! This video has correct English captions. Click on the CC button at the bottom of the video to see them. For "How Radiation Works - Americium 241, Alpha Particles and Gamma Rays", see: http://youtu.be/aJkx6hAD-4E For "How to make solar cells (DIY/homemade solar cell)", see: http://youtu.be/g5Edw99PgzQ For "Fresnel lens - what is it, testing focal length, solar heat generated", see: http://youtu.be/11n0ZaZMj3A To follow me on Twitter: https://twitter.com/#!/RimStarz http://rimstar.org 3...
published: 21 Dec 2012 -

Atoms and Their Electrons
Eduction video from 1996 If you want to see more videos like this, click that like button and subscribe button!!!
published: 03 Dec 2015 -

Electron Configuration
005 - Electron Configuration In this video Paul Andersen explains how to write out the electron configuration for atoms on the periodic table. More importantly he shows you why electrons arrange themselves in shells, subshells and orbitals by using Coulomb's law and studying the first ionization energies of different atoms. ANSWERS: Cl - [Ne] 3s^2 3p^5 Ag - [Kr] 4d^10 5s^1 - Did you get [Kr] 5s^2 4d^9? There are a few exceptions to this law. Most of them are found in the f-block metals and they are not of much chemical significance. Music Attribution Title: String Theory Artist: Herman Jolly http://sunsetvalley.bandcamp.com/track/string-theory All of the images are licensed under creative commons and public domain licensing: File:Electron Configuration Diagrams from H to Ne.svg,...
published: 04 Aug 2013 -

Discovery of the Electron: Cathode Ray Tube Experiment
To see all my Chemistry videos, check out http://socratic.org/chemistry J.J. Thompson discovered the electron, the first of the subatomic particles, using the cathode ray tube experiment. He found that many different metals release cathode rays, and that cathode rays were made of electrons, very small negatively charged particles. This disproved John Dalton's theory of the atom, and Thompson came up with the plum pudding model of the atom.
published: 28 Nov 2012 -

How Electrons Become Electricity
Electricity is the flow of electrons. This video shows how electrons move and become electricity using a power supply. This video was made as a part of the Quarked! website, a grant funded collaboration between the University of Kansas Department of Physics and Astronomy researchers and the educators at the Natural History Museum http://naturalhistory.ku.edu/. For more information, videos, and games, please visit http://www.quarked.org. © 2011 The University of Kansas
published: 28 Oct 2011 -

The Science of Electrons - What is an Electron?
Find me Around the Web: FACEBOOK - https://www.facebook.com/mrstargazer.nation TWITTER - https://twitter.com/STARGAZERNATION Blogspot - http://stargazernation.blogspot.co.uk/ On Pinterest: http://www.pinterest.com/Stargazernation/ Check out the latest: http://checkoutthelatest.com/MRSTARGAZERNATION Frequency: http://www.frequency.com/mrstargazernation?cid=4-2155340 Ashtar Command Crew: http://www.ashtarcommandcrew.net/profiles/blog/list?user=251glgsbn5l1o YouTube: https://www.youtube.com/user/MRSTARGAZERNATION Science Stage: http://sciencestage.com/stargazernation Before its News: http://beforeitsnews.com/contributor/pages/343/420/stories.html Blogster - http://www.blogster.com/stargazernation/
published: 07 May 2014
The Electron: Crash Course Chemistry #5
- Order: Reorder
- Duration: 12:48
- Updated: 12 Mar 2013
- views: 1310538
- published: 12 Mar 2013
- views: 1310538
Electrons, Protons And Neutrons | Standard Model Of Particle Physics
- Order: Reorder
- Duration: 7:44
- Updated: 19 Nov 2009
- views: 566544
- published: 19 Nov 2009
- views: 566544
Scientists in Sweden film moving electron for the first time
- Order: Reorder
- Duration: 0:27
- Updated: 26 Feb 2008
- views: 445044
- published: 26 Feb 2008
- views: 445044
Valence Electrons and the Periodic Table
- Order: Reorder
- Duration: 16:53
- Updated: 19 Dec 2012
- views: 451317
- published: 19 Dec 2012
- views: 451317
How Fast is an Electron and Electricity
- Order: Reorder
- Duration: 3:06
- Updated: 21 Dec 2012
- views: 150307
- published: 21 Dec 2012
- views: 150307
Atoms and Their Electrons
- Order: Reorder
- Duration: 34:26
- Updated: 03 Dec 2015
- views: 15606
- published: 03 Dec 2015
- views: 15606
Electron Configuration
- Order: Reorder
- Duration: 10:17
- Updated: 04 Aug 2013
- views: 817637
- published: 04 Aug 2013
- views: 817637
Discovery of the Electron: Cathode Ray Tube Experiment
- Order: Reorder
- Duration: 11:08
- Updated: 28 Nov 2012
- views: 220180
- published: 28 Nov 2012
- views: 220180
How Electrons Become Electricity
- Order: Reorder
- Duration: 1:31
- Updated: 28 Oct 2011
- views: 48940
- published: 28 Oct 2011
- views: 48940
The Science of Electrons - What is an Electron?
- Order: Reorder
- Duration: 1:25
- Updated: 07 May 2014
- views: 13241
- published: 07 May 2014
- views: 13241

- Playlist
- Chat

- Playlist
- Chat

The Electron: Crash Course Chemistry #5
- Report rights infringement
- published: 12 Mar 2013
- views: 1310538

Electrons, Protons And Neutrons | Standard Model Of Particle Physics
- Report rights infringement
- published: 19 Nov 2009
- views: 566544

Scientists in Sweden film moving electron for the first time
- Report rights infringement
- published: 26 Feb 2008
- views: 445044

Valence Electrons and the Periodic Table
- Report rights infringement
- published: 19 Dec 2012
- views: 451317

How Fast is an Electron and Electricity
- Report rights infringement
- published: 21 Dec 2012
- views: 150307

Atoms and Their Electrons
- Report rights infringement
- published: 03 Dec 2015
- views: 15606

Electron Configuration
- Report rights infringement
- published: 04 Aug 2013
- views: 817637

Discovery of the Electron: Cathode Ray Tube Experiment
- Report rights infringement
- published: 28 Nov 2012
- views: 220180

How Electrons Become Electricity
- Report rights infringement
- published: 28 Oct 2011
- views: 48940

The Science of Electrons - What is an Electron?
- Report rights infringement
- published: 07 May 2014
- views: 13241



