AM404
 |
|
| Identifiers | |
|---|---|
|
|
| CAS Number | 183718-77-6 |
| PubChem (CID) | 6604822 |
| ChemSpider | 5037081 |
| ChEMBL | CHEMBL39878 |
| Chemical and physical data | |
| Formula | C26H37NO2 |
| Molar mass | 395.577 g/mol |
| 3D model (Jmol) | Interactive image |
|
|
|
|
| |
|
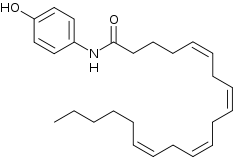
AM404, also known as N-arachidonoylaminophenol,[1] is an active metabolite of paracetamol (acetaminophen), responsible for all or part of its analgesic action.[2] Chemically, it is the amide formed from 4-aminophenol and arachidonic acid.
Pharmacology[edit]
AM404 was originally reported to be an endogenous cannabinoid reuptake inhibitor, preventing the transport of anandamide and other related compounds back from the synaptic cleft, much in the same way that common selective serotonin reuptake inhibitor (SSRI) antidepressants prevent the reuptake of serotonin. Earlier work on the mechanism of AM404 suggested that the inhibition of fatty acid amide hydrolase (FAAH) by AM404 was responsible for all of its attributed reuptake properties, since intracellular FAAH hydrolysis of anandamide changes the intra/extracellular anandamide equilibrium.[3] However, this is not the case, as newer research on FAAH knockout mice has found that brain cells internalize anandamide through a selective transport mechanism which is independent of FAAH activity.[4] This mechanism is inhibited by AM404.
AM404 is also a TRPV1 agonist and inhibitor of cyclooxygenase COX-1 and COX-2, thus attenuating prostaglandin synthesis. AM404 is thought to induce its analgesic action through its activity on the endocannabinoid, COX, and TRPV systems, all of which are present in pain and thermoregulatory pathways.[5]
See also[edit]
References[edit]
- ^ Rogosch T, Sinning C, Podlewski A, Watzer B, Schlosburg J, Lichtman AH, Cascio MG, Bisogno T, Di Marzo V, Nüsing R, Imming P (January 2012). "Novel bioactive metabolites of dipyrone (metamizol)". Bioorg. Med. Chem. 20 (1): 101–7. doi:10.1016/j.bmc.2011.11.028. PMC 3248997
 . PMID 22172309.
. PMID 22172309. - ^ Ottani A, Leone S, Sandrini M, Ferrari A, Bertolini A (February 2006). "The analgesic activity of paracetamol is prevented by the blockade of cannabinoid CB1 receptors". Eur. J. Pharmacol. 531 (1–3): 280–1. doi:10.1016/j.ejphar.2005.12.015. PMID 16438952.
- ^ Glaser ST, Abumrad NA, Fatade F, Kaczocha M, Studholme KM, Deutsch DG (April 2003). "Evidence against the presence of an anandamide transporter". Proc. Natl. Acad. Sci. U.S.A. 100 (7): 4269–74. doi:10.1073/pnas.0730816100. PMC 153082
 . PMID 12655057.
. PMID 12655057. - ^ Fegley, D.; Kathuria, S.; Mercier, R.; Li, C.; Goutopoulos, A.; Makriyannis, A.; Piomelli, D. (11 May 2004). "Anandamide transport is independent of fatty-acid amide hydrolase activity and is blocked by the hydrolysis-resistant inhibitor AM1172". Proceedings of the National Academy of Sciences. 101 (23): 8756–8761. doi:10.1073/pnas.0400997101. PMC 423268
 . PMID 15138300.
. PMID 15138300. - ^ Högestätt ED, Jönsson BA, Ermund A, Andersson DA, Björk H, Alexander JP, Cravatt BF, Basbaum AI, Zygmunt PM (September 2005). "Conversion of acetaminophen to the bioactive N-acylphenolamine AM404 via fatty acid amide hydrolase-dependent arachidonic acid conjugation in the nervous system" (pdf). J. Biol. Chem. 280 (36): 31405–12. doi:10.1074/jbc.M501489200. PMID 15987694.
| This drug article relating to the nervous system is a stub. You can help Wikipedia by expanding it. |