Cannabigerol
 |
|
 |
|
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C21H32O2 |
| Molar mass | 316.49 g·mol−1 |
| 3D model (Jmol) | |
|
|
|
|
| |
|
Cannabigerol (CBG) is a non-intoxicating cannabinoid found in the Cannabis genus of plants. CBG is the non-acidic form of cannabigerolic acid (CBGA), the parent molecule (“mother cannabinoid”) from which many other cannabinoids are made. By the time most strains of cannabis reach maturity, most of the CBG has been converted into other cannabinoids, primarily tetrahydrocannabinol (THC) or cannabidiol (CBD), usually leaving somewhere below 1% CBG in the plant.
CBG has been found to act as a high affinity α2-adrenergic receptor agonist, moderate affinity 5-HT1A receptor antagonist, and low affinity CB1 receptor antagonist.[1] It also binds to the CB2 receptor as an antagonist.[2] CBG does not trigger THC-like activity in mice, rats, gerbils and non-human primates, consistent with it being non-intoxicating.[3] [4] Moreover, CBG was without effect up to 80 mg/kg in the mouse tetrad test of cannabimimetic activity (locomotor suppression, catalepsy, hypothermia and analgesia).[5]
Contents
Chemistry[edit]
It has two E/Z isomers.
Potential uses[edit]
Pain, anxiety[edit]
CBG has potential for alleviating pain,[6] especially neuropathic pain where tests suggest a higher efficacy than CBD[7] CBG can also inhibit the uptake of GABA in the brain, which can decrease anxiety and muscle tension[8] with tests on mice showing that CBG induces antidepressant effects similar to imipramine.[9]
Inflammation, digestive conditions[edit]
It has been shown to improve a model of inflammatory bowel disease,[10] ulcerative colitis and Crohn's disease.[11]
Skin conditions[edit]
CBG induces production of the body’s natural skin moisturizers, holding promise for dry-skin syndromes and with the potential to treat other skin conditions.[12]
Glaucoma[edit]
Cannabigerol has been shown to relieve intraocular pressure, which may be of benefit in the treatment of glaucoma.[13][14]
Neuroprotection[edit]
CBG has been shown to have neuroprotective properties and may prove promising for the treatment of neurodegenerative diseases such as Huntington’s disease[15] and Multiple Sclerosis.[16]
Antiseptic[edit]
CBG is known to have antiseptic properties[17][18] and research suggests that it might be effective against the superbug MRSA.[19]
Cancer[edit]
CBG is showing promising properties in vitro for the potential treatment of a broad range of cancers including breast, liver, lung, pancreatic, skin, ovarian, renal, bladder and colon cancer.[20][21][22][23][24]
Legal status[edit]
CBG is not scheduled by Convention on Psychotropic Substances.
United States[edit]
CBG is not scheduled at the federal level in the United States.[25]
Biosynthesis[edit]
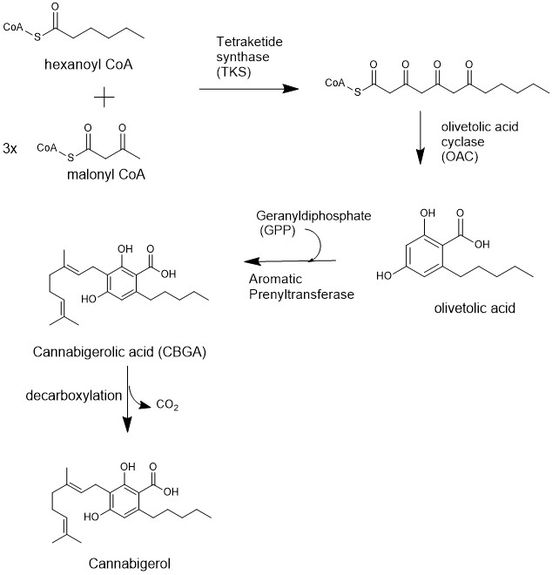
The biosynthesis of CBG begins by loading a hexanoyl CoA onto a ketide synthetase assembly protein and subsequent condensation with three malonyl CoA molecules.[26] This polyketide is cyclized to olivetolic acid via an olivetolic cyclase, and then prenylated with a 10 carbon isoprenoid precursor, geranyldiphosphate (GPP), using an aromatic prenyltransferase enzyme to biosynthesize cannabigerolic acid (CBGA), which can then be decarboxylated to yield cannabigerol.[27]
See also[edit]
References[edit]
- ^ Cascio MG, Gauson LA, Stevenson LA, Ross RA, Pertwee R (December 2009). "Evidence that the plant cannabinoid cannabigerol is a highly potent alpha(2)-adrenoceptor agonist and moderately potent 5HT receptor antagonist". British Journal of Pharmacology. 159 (1): 129–141. doi:10.1111/j.1476-5381.2009.00515.x. PMC 2823359
 . PMID 20002104.
. PMID 20002104. - ^ Cascio MG, Gauson LA, Stevenson LA, Ross RA, & Pertwee RG (2010). Evidence that the plant cannabinoid cannabigerol is a highly potent α2-adrenoceptor agonist and moderately potent 5HT1A receptor antagonist
- ^ Grunfeld Y, & Edery H (1969). Psychopharmacological activity of the active constituents of hashish and some related cannabinoids
- ^ Mechoulam R, Shani A, Edery H, & Grunfeld Y (1970). Chemical basis of hashish activity
- ^ El-Alfy AT, Ivey K, Robinson K, Ahmed S, Radwan M, Slade D, et al. (2010). Antidepressant-like effect of Δ9-tetrahydrocannabinol and other cannabinoids isolated from Cannabis sativa L
- ^ Ethan B Russo (2011). Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects(p1348)
- ^ Sabatino Maione, Francesco Rossi, Geoffrey Guy, Colin Stott, Tetsuro Kikuchi (2011). Cannabinoids for use in the treatment of neuropathic pain
- ^ Medical Jane, Cannabigerol (CBG): A Minor Cannabinoid With A Major Impact
- ^ Richard Musty, Richard Deyo (2006). Pharmaceutical compositions comprising cannabigerol
- ^ Borrelli, F; Fasolino, I; Romano, B; Capasso, R; Maiello, F; Coppola, D; Orlando, P; Battista, G; Pagano, E; Di Marzo, V; Izzo, AA (May 2013). "Beneficial effect of the non-psychotropic plant cannabinoid cannabigerol on experimental inflammatory bowel disease.". Biochem Pharmacol. 85 (9): 1306–16. doi:10.1016/j.bcp.2013.01.017. PMID 23415610.
- ^ Angelo Antonio Izzo, Francesca Borrelli, Stephen Wright (2011). Phytocannabinoids for use in the treatment of intestinal inflammatory diseases
- ^ Oláh A, Markovics A, Szabó-Papp J, Szabó PT, Stott C, Zouboulis CC, Bíró T (2016). Differential effectiveness of selected non-psychotropic phytocannabinoids on human sebocyte functions implicates their introduction in dry/seborrhoeic skin and acne treatment
- ^ Colasanti, B. (1990). "A comparison of the ocular and central effects of delta 9-tetrahydrocannabinol and cannabigerol". Journal of ocular pharmacology. 6 (4): 259–269. doi:10.1089/jop.1990.6.259. PMID 1965836.
- ^ Colasanti, B.; Craig, C.; Allara, R. (1984). "Intraocular pressure, ocular toxicity and neurotoxicity after administration of cannabinol or cannabigerol". Experimental eye research. 39 (3): 251–259. doi:10.1016/0014-4835(84)90013-7. PMID 6499952.
- ^ Valdeolivas S, Navarrete C, Cantarero I, Bellido ML, Muñoz E, Sagredo O (2015). Neuroprotective properties of cannabigerol in Huntington's disease: studies in R6/2 mice and 3-nitropropionate-lesioned mice
- ^ Granja AG, Carrillo-Salinas F, Pagani A, Gómez-Cañas M, Negri R, Navarrete C, Mecha M, Mestre L, Fiebich BL, Cantarero I, Calzado MA, Bellido ML, Fernandez-Ruiz J, Appendino G, Guaza C, Muñoz E (2012). A cannabigerol quinone alleviates neuroinflammation in a chronic model of multiple sclerosis
- ^ Hala N. Eisohly, Carlton E. Turner, Alice M. Clark,Mahmoud A. Eisohly (1982). Synthesis and antimicrobial activities of certain cannabichromene and cannabigerol related compounds
- ^ George ANASTASSOV, Lekhram Changoer (2014). Oral care composition comprising cannabinoids
- ^ Appendino G1, Gibbons S, Giana A, Pagani A, Grassi G, Stavri M, Smith E, Rahman MM (2008). Antibacterial cannabinoids from Cannabis sativa: a structure-activity study
- ^ Colin Stott, Marnie DUNCAN, Thomas Hill (2014). Active pharmaceutical ingredient (api) comprising cannabinoids for use in the treatment of cancer
- ^ Ethan B Russo (2011). Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects(p 1348)
- ^ Seung-Hwa Baek, Seok Du Han, Chan Nam Yook, Young Chae Kim, Jung Suk Kwak (1996). Synthesis and antitumor activity of cannabigerol
- ^ Seung-Hwa Baek, Seok Du Han, Chan Nam Yook, Young Chae Kim, Jung Suk Kwak (1998). Boron trifluoride etherate on silica-A modified lewis acid reagent (VII). Antitumor activity of cannabigerol against human oral epitheloid carcinoma cells
- ^ Borrelli F, Pagano E, Romano B, Panzera S, Maiello F, Coppola D, De Petrocellis L, Buono L, Orlando P, Izzo AA (2014). Colon carcinogenesis is inhibited by the TRPM8 antagonist cannabigerol, a Cannabis-derived non-psychotropic cannabinoid
- ^ §1308.11 Schedule I.
- ^ Page Et. Al, Jonathan (2012). "Identification of olivetolic acid cyclase from Cannabis sativa reveals a unique catalytic route to plant polyketides". PNAS. 109: 12811–6. doi:10.1073/pnas.1200330109. PMID 22802619.
- ^ Meinhart H,, Zenk Et. Al (1998). "Prenylation of olivetolate by a hemp transferase yields cannabigerolic acid, the precursor of tetrahydrocannabinol". FEBS Letters. 427: 283–285. doi:10.1016/s0014-5793(98)00450-5. PMID 9607329.