Enadoline
From Wikipedia, the free encyclopedia
 |
|
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
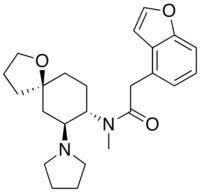
| Formula | C24H32N2O3 |
| Molar mass | 396.52 g/mol |
| 3D model (Jmol) | |
|
|
|
|
| (verify) | |
Enadoline is a drug which acts as a highly selective κ-opioid agonist.
In human studies, it produced visual distortions and feelings of dissociation, reminiscent of the effects of salvinorin A.[1]
It was studied as a potential analgesic, but abandoned because of the dose-limiting effects of dysphoria, which could be expected from a κ-opioid agonist. There was mention of its potential in treating comatose head injury or stroke victims, where that type of side effect would be immaterial.[2]
Potency[edit]
When enadoline was first reported in 1990, it was "the most potent κ-selective analgesic ever reported ... 25 times more potent than morphine and 17 times more potent than U-62066".[3]
References[edit]
- ^ Walsh SL, Strain EC, Abreu ME, Bigelow GE (2001). "Enadoline, a selective kappa opioid agonist: comparison with butorphanol and hydromorphone in humans". Psychopharmacology (Berl.). 157 (2): 151–62. doi:10.1007/s002130100788. PMID 11594439.
- ^ Barber A, Gottschlich R (1997). "Novel developments with selective, non-peptidic kappa-opioid receptor agonists". Expert Opin Investig Drugs. 6 (10): 1351–68. doi:10.1517/13543784.6.10.1351. PMID 15989506.
- ^ Halfpenny, Paul R.; Horwell, David C.; Hughes, John; Hunter, John C.; Rees, David C. (1990). "Highly selective .kappa.-opioid analgesics. 3. Synthesis and structure-activity relationships of novel N-[2-(1-pyrrolidinyl)-4- or -5-substituted cyclohexyl]arylacetamide derivatives". Journal of Medicinal Chemistry. 33 (1): 286–91. doi:10.1021/jm00163a047. PMID 2153208.
| This psychoactive drug-related article is a stub. You can help Wikipedia by expanding it. |