Ibipinabant
From Wikipedia, the free encyclopedia
 |
|
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.158.931 |
| Chemical and physical data | |
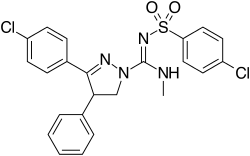
| Formula | C24H22Cl2N4O2S |
| Molar mass | 501.427 |
| 3D model (Jmol) | |
|
|
|
|
| |
|
Ibipinabant (SLV319, BMS-646,256) is a drug used in scientific research which acts as a potent and highly selective CB1 antagonist.[1] It has potent anorectic effects in animals,[2] and was researched for the treatment of obesity, although CB1 antagonists as a class have now fallen out of favour as potential anorectics following the problems seen with rimonabant, and so ibipinabant is now only used for laboratory research, especially structure-activity relationship studies into novel CB1 antagonists.[3][4][5]
See also[edit]
References[edit]
- ^ Lange, JH; Coolen, HK; Van Stuivenberg, HH; Dijksman, JA; Herremans, AH; Ronken, E; Keizer, HG; Tipker, K; et al. (2004). "Synthesis, biological properties, and molecular modeling investigations of novel 3,4-diarylpyrazolines as potent and selective CB(1) cannabinoid receptor antagonists". Journal of Medicinal Chemistry. 47 (3): 627–43. doi:10.1021/jm031019q. PMID 14736243.
- ^ Need, AB; Davis, RJ; Alexander-Chacko, JT; Eastwood, B; Chernet, E; Phebus, LA; Sindelar, DK; Nomikos, GG (2006). "The relationship of in vivo central CB1 receptor occupancy to changes in cortical monoamine release and feeding elicited by CB1 receptor antagonists in rats". Psychopharmacology. 184 (1): 26–35. doi:10.1007/s00213-005-0234-x. PMID 16328376.
- ^ Lange, JH; Van Stuivenberg, HH; Veerman, W; Wals, HC; Stork, B; Coolen, HK; McCreary, AC; Adolfs, TJ; Kruse, CG (2005). "Novel 3,4-diarylpyrazolines as potent cannabinoid CB1 receptor antagonists with lower lipophilicity". Bioorganic & Medicinal Chemistry Letters. 15 (21): 4794–8. doi:10.1016/j.bmcl.2005.07.054. PMID 16140010.
- ^ Srivastava, BK; Joharapurkar, A; Raval, S; Patel, JZ; Soni, R; Raval, P; Gite, A; Goswami, A; et al. (2007). "Diaryl dihydropyrazole-3-carboxamides with significant in vivo antiobesity activity related to CB1 receptor antagonism: synthesis, biological evaluation, and molecular modeling in the homology model". Journal of Medicinal Chemistry. 50 (24): 5951–66. doi:10.1021/jm061490u. PMID 17979261.
- ^ Srivastava, BK; Soni, R; Joharapurkar, A; Sairam, KV; Patel, JZ; Goswami, A; Shedage, SA; Kar, SS; et al. (2008). "Bioisosteric replacement of dihydropyrazole of 4S-(−)-3-(4-chlorophenyl)-N-methyl-N'-(4-chlorophenyl)-sulfonyl-4-phenyl-4,5-dihydro-1H-pyrazole-1-caboxamidine (SLV-319) a potent CB1 receptor antagonist by imidazole and oxazole". Bioorganic & Medicinal Chemistry Letters. 18 (3): 963–8. doi:10.1016/j.bmcl.2007.12.036. PMID 18207393.
| This cannabinoid related article is a stub. You can help Wikipedia by expanding it. |