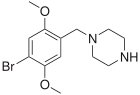
2C-B-BZP
 |
|
 |
|
| Identifiers | |
|---|---|
|
|
| CAS Number | 1094424-37-9 |
| ChemSpider | 26234933 |
| UNII | R0E29C6K2K |
| Chemical and physical data | |
| Formula | C13H19BrN2O2 |
| Molar mass | 315.2085 g/mol |
| 3D model (Jmol) | Interactive image |
|
|
|
|
| |
|
4-Bromo-2,5-dimethoxy-1-benzylpiperazine (2C-B-BZP) is a psychoactive drug and research chemical of the piperazine chemical class which has been sold as a "designer drug".[1][2] It produces stimulant effects similar to those of benzylpiperazine (BZP).
Contents
Chemistry[edit]
2C-B-BZP contains a benzylpiperazine base as well as the ring-substitution pattern of the psychedelic phenethylamine 2C-B. 2C-B-BZP is not a phenethylamine itself and does not produce psychedelic effects, as the binding groups are in the wrong position to activate the 5-HT2A receptor, while 2C-B-phenylpiperazine substitutes for DOM in DOM-trained rats with around 1/10th the potency of DOM, but does not substitute for TFMPP.[3]:867–868
Effects[edit]
2C-B-BZP produces stimulant effects which last 3–6 hours. It is also said by several sources to increase the effects of other compounds when combined[citation needed]. Side effects include headaches and nausea, similar to those of other recreationally-used piperazine derivatives.
Legality[edit]
2C-B-BZP is unscheduled and uncontrolled in the United States, but possession and sale of 2C-B-BZP could possibly be prosecuted under the Federal Analog Act because of its structural similarities to benzylpiperazine. 2C-B-BZP is illegal to possess, use or sell in Japan where it used to be sold in local smartshops.
See also[edit]
- 1-Benzylpiperazine (BZP)
- 1-Methyl-4-benzylpiperazine (MBZP)
- 1,4-Dibenzylpiperazine (DBZP)
- 3-Chlorophenylpiperazine (mCPP)
- 3-Trifluoromethylphenylpiperazine (TFMPP)
- 3,4-Methylenedioxy-1-benzylpiperazine (MDBZP)
- 4-Fluorophenylpiperazine (pFPP)
- 4-Methoxyphenylpiperazine (MeOPP)
References[edit]
- ^ Westphal F, Junge T, Girreser U, Stobbe S, Pérez SB (May 2009). "Structure elucidation of a new designer benzylpiperazine: 4-bromo-2,5-dimethoxybenzylpiperazine". Forensic Science International. 187 (1-3): 87–96. doi:10.1016/j.forsciint.2009.03.003. PMID 19345524.
- ^ Peters FT, Martinez-Ramirez JA (October 2010). "Analytical toxicology of emerging drugs of abuse". Therapeutic Drug Monitoring. 32 (5): 532–9. doi:10.1097/FTD.0b013e3181f33411. PMID 20814349.
- ^ Daniel Trachsel; David Lehmann & Christoph Enzensperger (2013). Phenethylamine: Von der Struktur zur Funktion. Nachtschatten Verlag AG. ISBN 978-3-03788-700-4.