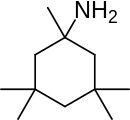
Neramexane is a drug related to memantine ,[1] NMDA antagonist [2] neuroprotective effects.[3] tinnitus ,[4] [5] Alzheimer's disease ,[6] drug addiction [7] analgesic .[8] antidepressant [9] nootropic [10] nicotinic acetylcholine receptor antagonist .[11]
A clinical trial found that doses of 50mg and above safely improved tinnitus scores over 16 weeks.[12]
See also [ edit ] References [ edit ]
^ Gilling, K; Jatzke, C; Wollenburg, C; Vanejevs, M; Kauss, V; Jirgensons, A; Parsons, CG (2007). "A novel class of amino-alkylcyclohexanes as uncompetitive, fast, voltage-dependent, N-methyl-D-aspartate (NMDA) receptor antagonists--in vitro characterization". Journal of Neural Transmission . 114 (12): 1529–37. doi :10.1007/s00702-007-0792-7 . PMID 17728997 . ^ Danysz, W; Parsons, CG; Jirgensons, A; Kauss, V; Tillner, J (2002). "Amino-alkyl-cyclohexanes as a novel class of uncompetitive NMDA receptor antagonists". Current Pharmaceutical Design . 8 (10): 835–43. doi :10.2174/1381612024607117 . PMID 11945134 . ^ Danysz, W; Parsons, CG (Mar 2002). "Neuroprotective potential of ionotropic glutamate receptor antagonists". Neurotoxicity Research . 4 (2): 119–26. doi :10.1080/10298420290015872 . PMID 12829411 . ^ Clinical trial number NCT00405886 ClinicalTrials.gov ^ Clinical trial number NCT00739635 ClinicalTrials.gov ^ Rammes, G; Schierloh, A (Feb 2006). "Neramexane (merz pharmaceuticals/forest laboratories)". IDrugs . 9 (2): 128–35. PMID 16523403 . ^ Kotlinska, J; Biala, G; Rafalski, P; Bochenski, M; Danysz, W (Oct 2004). "Effect of neramexane on ethanol dependence and reinforcement". European Journal of Pharmacology . 503 (1-3): 95–8. doi :10.1016/j.ejphar.2004.09.036 . PMID 15496302 . ^ Klein, T; Magerl, W; Hanschmann, A; Althaus, M; Treede, RD (Jan 2008). "Antihyperalgesic and analgesic properties of the N-methyl-D-aspartate (NMDA) receptor antagonist neramexane in a human surrogate model of neurogenic hyperalgesia". European Journal of Pain . 12 (1): 17–29. doi :10.1016/j.ejpain.2007.02.002 . PMID 17449306 . ^ Kos, T; Legutko, B; Danysz, W; Samoriski, G; Popik, P (Sep 2006). "Enhancement of antidepressant-like effects but not brain-derived neurotrophic factor mRNA expression by the novel N-methyl-D-aspartate receptor antagonist neramexane in mice". Journal of Pharmacology and Experimental Therapeutics . 318 (3): 1128–36. doi :10.1124/jpet.106.103697 . PMID 16740621 . ^ Zoladz, PR; Campbell, AM; Park, CR; Schaefer, D; Danysz, W; Diamond, DM (Oct 2006). "Enhancement of long-term spatial memory in adult rats by the noncompetitive NMDA receptor antagonists, memantine and neramexane". Pharmacology, Biochemistry and Behaviour . 85 (2): 298–306. doi :10.1016/j.pbb.2006.08.011 . PMID 17045636 . ^ Plazas PV, Savino J, Kracun S, et al. (July 2007). "Inhibition of the alpha9alpha10 nicotinic cholinergic receptor by neramexane, an open channel blocker of N-methyl-D-aspartate receptors" . European Journal of Pharmacology . 566 (1-3): 11–9. doi :10.1016/j.ejphar.2007.03.026 . ^ "A randomized, double-blind, placebo-controlled clinical trial to evaluate the efficacy and safety of neramexane in patients with moderate to severe subjective tinnitus.". BMC Ear, Nose and Throat Disorders . 11 : 1. Jan 2011. doi :10.1186/1472-6815-11-1 . PMID 21223542 .
Psychedelics (5-HT2A
HOT-*
25*-NB*(excludes FLY)
Subst.
3C-*
4C-*
FLY
Others
4,5-DHP-DMT 2,N,N-TMT 4-AcO-DMT 4-HO-5-MeO-DMT 4,N,N-TMT 4-Propionyloxy-DMT 5,6-diBr-DMT 5-AcO-DMT 5-Bromo-DMT 5-MeO-2,N ,N -TMT 5-MeO-4,N ,N -TMT 5-MeO-α,N,N-TMT 5-MeO-DMT 5-N ,N -TMT 7,N,N-TMT α,N,N-TMT (Bufotenin) 5-HO-DMT DMT Norbaeocystin (Psilocin) 4-HO-DMT (Psilocybin) 4-PO-DMT
Others
Others
Dissociatives (NMDAR antagonists )
Deliriants (mAChR antagonists )
Others
Non-selective
MAOA -selective
MAOB -Selective
Atypical antipsychotics (aripiprazole , lurasidone , olanzapine , quetiapine , lurasidone )Lithium (lithium carbonate , lithium citrate )Thyroid hormones (triiodothyronine (T3 ), levothyroxine (T4 ))NMDA receptor antagonists (esketamine , ketamine , pregabalin , gabapentin , dextromethorphan , memantine , amantadine )
mACh
Muscarinic antagonists :3-Quinuclidinyl benzilate 4-DAMP Aclidinium bromide Anisodamine Anisodine Antihistamines (first-generation) (e.g., brompheniramine , chlorphenamine , cyproheptadine , dimenhydrinate , diphenhydramine , doxylamine , mepyramine (pyrilamine) , phenindamine , pheniramine , promethazine , tripelennamine , triprolidine )Atropine Atropine methonitrate Atypical antipsychotics (e.g., clozapine , olanzapine , quetiapine , zotepine )Benactyzine Benzatropine (benztropine) Benzilylcholine mustard Benzydamine BIBN 99 Biperiden Bornaprine CAR-226,086 CAR-301,060 CAR-302,196 CAR-302,282 CAR-302,368 CAR-302,537 CAR-302,668 Caramiphen Cloperastine CS-27349 Cyclobenzaprine Cyclopentolate Darifenacin DAU-5884 Dimethindene Dexetimide DIBD Dicyclomine (dicycloverine) Ditran EA-3167 EA-3443 EA-3580 EA-3834 Etanautine Etybenzatropine (ethybenztropine) Flavoxate Himbacine HL-031,120 Ipratropium bromide J-104,129 Hyoscyamine Mamba toxin 3 Mamba toxin 7 Mazaticol Mebeverine Methoctramine Metixene N-Ethyl-3-piperidyl benzilate N-Methyl-3-piperidyl benzilate Orphenadrine Otenzepad Oxybutynin PBID PD-102,807 PD-0298029 Phenglutarimide Phenyltoloxamine Pipenzolate bromide Pirenzepine Piroheptine Procyclidine Profenamine Revefenacin RU-47,213 SCH-57,790 SCH-72,788 SCH-217,443 Scopolamine (hyoscine) Sofpironium bromide Solifenacin Telenzepine Tetracyclic antidepressants (e.g., amoxapine , maprotiline , mianserin , mirtazapine )Timepidium bromide Tiotropium bromide Tolterodine Tricyclic antidepressants (e.g., amitriptyline , butriptyline , clomipramine , desipramine , dosulepin (dothiepin) , doxepin , imipramine , lofepramine , nortriptyline , protriptyline , trimipramine )Trihexyphenidyl Tripitamine Tropacine Tropatepine Tropicamide Typical antipsychotics (e.g., chlorpromazine , loxapine , thioridazine )WIN-2299 Xanomeline Zamifenacin
nACh
Nicotinic agonists :5-HIAA A-84,543 A-366,833 A-582,941 A-867,744 ABT-202 ABT-418 ABT-560 ABT-894 Acetylcholine Altinicline Anabasine Anatoxin-a AR-R17779 Butinoline Butyrylcholine Carbachol Choline Cotinine Cytisine Decamethonium Desformylflustrabromine Dianicline Dimethylphenylpiperazinium Epibatidine Epiboxidine Ethanol Ethoxysebacylcholine EVP-4473 EVP-6124 Galantamine GTS-21 Ispronicline Ivermectin Levamisole Lobeline MEM-63,908 (RG-3487) Morantel Nicotine (tobacco )NS-1738 PHA-543,613 PHA-709,829 PNU-120,596 PNU-282,987 Pozanicline Rivanicline RJR-2429 Sazetidine A SB-206553 Sebacylcholine SIB-1508Y SIB-1553A SSR-180,711 Suberyldicholine Suxamethonium (succinylcholine) TC-1698 TC-1734 TC-1827 TC-2216 TC-5214 TC-5619 TC-6683 Tebanicline Tropisetron UB-165 Varenicline WAY-317,538 XY-4083
Receptor (ligands )
AMPA
NMDA
Antagonists: Competitive antagonists: AP5 (APV) AP7 CGP-37849 CGP-39551 CGP-39653 CGP-40116 CGS-19755 CPP LY-233,053 LY-235,959 LY-274,614 MDL-100,453 Midafotel (d-CPPene) NPC-12,626 NPC-17,742 PBPD PEAQX Perzinfotel PPDA SDZ-220581 Selfotel ; Noncompetitive antagonists: ARR-15,896 Caroverine Dexanabinol FPL-12495 FR-115,427 Hodgkinsine Magnesium MDL-27,266 NPS-1506 Psychotridine Zinc ; Uncompetitive pore blockers: 2-MDP 3-HO-PCP 3-MeO-PCE 3-MeO-PCMo 3-MeO-PCP 4-MeO-PCP 8A-PDHQ 18-MC α-Endopsychosin Alaproclate Amantadine Aptiganel Arketamine ARL-12,495 ARL-15,896-AR ARL-16,247 Budipine Conaridine Delucemine Dexoxadrol Dextrallorphan Dieticyclidine Diphenidine Dizocilpine Ephenidine Esketamine Etoxadrol Eticyclidine Fluorolintane Gacyclidine Ibogaine Ibogamine Indantadol Ketamine Ketobemidone Lanicemine Loperamide Memantine Methadone (Levomethadone )Methorphan (Dextromethorphan Levomethorphan )Methoxetamine Methoxphenidine Milnacipran Morphanol (Dextrorphan Levorphanol )NEFA Neramexane Nitromemantine Nitrous oxide Noribogaine Norketamine Orphenadrine PCPr Pethidine (meperidine) Phencyclamine Phencyclidine Propoxyphene Remacemide Rhynchophylline Rimantadine Rolicyclidine Sabeluzole Tabernanthine Tenocyclidine Tiletamine Tramadol Xenon ; Glycine site antagonists: 4-Cl-KYN (AV-101) 5,7-DCKA 7-CKA ACC ACEA-1011 ACEA-1328 AV-101 Carisoprodol CGP-39653 CNQX DNQX Felbamate Gavestinel GV-196,771 Kynurenic acid Kynurenine L-689,560 L-701,324 Licostinel (ACEA-1021) LU-73,068 MDL-105,519 Meprobamate MRZ 2/576 PNQX ZD-9379 ; NR2B subunit antagonists: Besonprodil CERC-301 (MK-0657) CO-101,244 (PD-174,494) Eliprodil Haloperidol Ifenprodil Isoxsuprine Nylidrin Ro8-4304 Ro25-6981 Traxoprodil ; Polyamine site antagonists: Arcaine Co 101676 Diaminopropane Diethylenetriamine Huperzine A Putrescine Ro 25-6981 ; Unclassified/unsorted antagonists: Bumetanide Chloroform Cyclopropane D -αAADiethyl ether Enflurane Ethanol Flufenamic acid Flupirtine Furosemide Halothane Isoflurane Metaphit Methoxyflurane Niflumic acid Pentamidine isethionate Piretanide Toluene Transcrocetin (saffron )Trichloroethane Trichloroethanol Trichloroethylene Xylene
Kainate
mGlu1
mGlu2
mGlu3
mGlu4
mGlu5
mGlu6
mGlu7
mGlu8
Transporter (blockers )
Enzyme (inhibitors )
Others