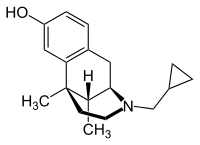
Cyclazocine
 |
|
| Clinical data | |
|---|---|
| Routes of administration |
Oral |
| ATC code |
|
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider |
|
| UNII | |
| KEGG |
|
| ChEMBL | |
| ECHA InfoCard | 100.028.060 |
| Chemical and physical data | |
| Formula | C18H25NO |
| Molar mass | 271.40 g/mol |
| 3D model (Jmol) | |
|
|
|
|
| |
|
Cyclazocine is a mixed opioid agonist/antagonist related to dezocine, pentazocine and phenazocine. This family of opioid drugs is called the benzomorphans or benzazocines.[1] It is a KOR agonist and MOR partial agonist, and also has high affinity for the DOR.[2]
Use[edit]
Research into the use of cyclazocine for the treatment of bipolar patients with depression was undertaken by Fink and colleagues (1970). It showed that 8 out of 10 patients experienced moderate improvement.
Research during the 1960s and 1970s into the possible use of cyclazocine for management of pain, and later for assisting treatment of narcotic addiction was severely hampered by the drug's psychotomimetic, dysphoric, and hallucinatory effects.[3] The dysphoric/anxiety inducing effects the drug correlate with increasing dosage and would likely reduce the risk of abuse in the same manner as other opioids which preferentially act on the KOR versus the DOR. and MOR, although the side-effect threshold is often lower than the lowest effective dose.
See also[edit]
References[edit]
- ^ Archer, S.; Glick, S. D.; Bidlack, J. M. (1996). "Cyclazocine Revisited". Neurochemical Research. 21 (11): 1369–1373. doi:10.1007/BF02532378. PMID 8947927.
- ^ Bidlack JM, Cohen DJ, McLaughlin JP, Lou R, Ye Y, Wentland MP (July 2002). "8-Carboxamidocyclazocine: a long-acting, novel benzomorphan". J. Pharmacol. Exp. Ther. 302 (1): 374–80. doi:10.1124/jpet.302.1.374. PMID 12065740.
- ^ Freedman, A. M.; Fink, M.; Sharoff, R.; Zaks, A. (1967). "Cyclazocine and Methadone in Narcotic Addiction" (pdf). The Journal of the American Medical Association. 202 (3): 191–194. doi:10.1001/jama.1967.03130160065011. PMID 6072354.
| Opioids | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Paracetamol-type | |||||||||||||||||||||||||||||||
| NSAIDs |
|
||||||||||||||||||||||||||||||
| Cannabinoids | |||||||||||||||||||||||||||||||
| Ion channel modulators |
|
||||||||||||||||||||||||||||||
| Myorelaxants | |||||||||||||||||||||||||||||||
| Others | |||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
| MOR |
|
|---|---|
| DOR |
|
| KOR |
|
| NOP |
|
| Unsorted | |
| Others |
|
|
See also: Peptide receptor modulators
|
|
| Agonists |
|
|---|---|
| Antagonists | |
| Unknown / unsorted |
|