Metrifonate
From Wikipedia, the free encyclopedia
(Redirected from Trichlorfon)
 |
|
| Clinical data | |
|---|---|
| Trade names | Chlorophos (and many others)[1]Trichlorfon; Phosphonic acid, (2,2,2-trichloro-1-hydroxyethyl)-, dimethyl ester; (2,2,2-Trichloro-1-hydroxyethyl) dimethylphosphonate; Agroforotox; Anthon; Chlorofos; Chloroftalm; Chlorophos; Chlorophthalm; Chloroxyphos; Combot; Dimethyl (trichlorohydroxyethyl)phosphonate; Dimethyl (1-hydroxy-2,2,2-trichloroethyl)phosphonate; Dimethyl (2,2,2-trichloro-1-hydroxyethyl)phosphonate; Dipterax; Dipterex; Dipterex 50; Diptevur; Ditrifon; Dylox; Dyrex; Dyvon; DEP; DEP (Pesticide); DETF; ENT 19,763; Flibol E; Fliegenteller; Forotox; Foschlor; Foschlor R; Foschlor R 50; Hypodermacid; Loisol; Masoten; Mazoten; Methyl Chlorophos; Metifonate; Metriphonate; Neguron; Neguvon; O,O-Dimethyl (1-hydroxy-2,2,2-trichloroethyl)phosphonate; O,O-Dimethyl (2,2,2-trichloro-1-hydroxyethyl)phosphonate; O,O-Dimethyl (2,2,2-trichlorohydroxyethyl)phosphonate; Phoschlor; Phoschlor R50; Polfoschlor; Ricifon; Ritsifon; Soldep; Sotipox; Trichlorphon; Trichlorphon FN; Tugon; Volfartol; Votexit; Wotexit; WEC 50; (1-Hydroxy-2,2,2-trichloroethyl)phosphonic acid, dimethyl ester; (2,2,2-Trichloro-1-hydroxyethyl)phosphonate, dimethyl ester; (2,2,2-Trichloro-1-hydroxyethyl)phosphonic acid dimethyl ester; Bayer L 1359; Bilarcil; Bovinox; Briton; BAY 15922; Cekufon; Chlorophosciclosom; Clorofos; Danex; Dimethoxy-(2,2,2-trichloro-1-hydroxyethyl)phosphine oxide; Equino-Aid; Foschlorem; Khloroftalm; NCI-C54831; O,O-Dimethyl (1-hydroxy-2,2,2-trichloroethyl)phosphate; O,O-Dimethyl-(1-hydroxy-2,2,2-trichloraethyl)phosphosaeure ester; O,O-Dimethyl-(1-hydroxy-2,2,2-trichlorathyl)-phosphat; O,O-Dimethyl-(2,2,2-trichloor-1-hydroxy-ethyl)-fosfonaat; O,O-Dimethyl-(2,2,2-trichlor-1-hydroxy-aethyl)phosphonat; O,O-Dimetil-(2,2,2-tricloro-1-idrossi-etil)-fosfonato; Phosphonic acid, (1-hydroxy-2,2,2-trichloroethyl)-, dimethyl ester; Proxol; Trichloorfon; Trichlorofon; Trichlorophon; Trinex; 1-Hydroxy-2,2,2-trichloro-ethyle phosphonate de dimethyle; Bay-L 1359; Briten; Denkaphon; Dipterex WP 80; Ertefon; OMS 800; Zeltivar; Onefon; O,O-Dimethyl-1-oxy-2,2,2-trichloroethyl phosphonate;Trichlorfon; 1-Hydroxy-2,2,2-trichloroethylphosphonate-O,O-dimethyl ester; Phosphonic acid, P-(2,2,2-trichloro-1-hydroxyethyl)-, dimethyl ester; Chlorak; Dimetox; Dioxaphos[2] |
| AHFS/Drugs.com | International Drug Names |
| ATC code | P02BB01 (WHO) QP52AB01 (WHO) QP53AF02 (WHO) |
| Pharmacokinetic data | |
| Biological half-life | 3 hours |
| Identifiers | |
|
|
| Synonyms | Trichlorphon |
| CAS Number | 52-68-6 |
| PubChem (CID) | 5853 |
| ChemSpider | 5644 |
| UNII | DBF2DG4G2K |
| KEGG | C07971 |
| ChEBI | CHEBI:6908 |
| ECHA InfoCard | 100.000.137 |
| Chemical and physical data | |
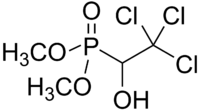
| Formula | C4H8Cl3O4P |
| Molar mass | 257.436 g/mol |
| 3D model (Jmol) | Interactive image |
| Chirality | Racemic mixture |
|
|
|
|
| |
|
Metrifonate (INN) or trichlorfon (USAN) is an irreversible organophosphate acetylcholinesterase inhibitor.[3] It is a prodrug which is activated non-enzymatically into 2,2-dichlorovinyl dimethyl phosphate (DDVP).
It is used as an insecticide.
It can be used to treat schistosomiasis[4] caused by Schistoma haematobium,[5] but is no longer commercially available.[6]
It has been proposed for use in treatment of Alzheimer's disease, but use for that purpose is not currently recommended.[7]
References[edit]
- ^ "Trichlorfon". Haz-Map. U.S. National Library of Medicine. August 2015. Retrieved 2015-10-13.
- ^ "Metrifonate". U.S. National Institute of Standards and Technology. Retrieved 2016-09-04.
- ^ "NLH - Neurological Conditions - Metrifonate for Alzheimer's disease".
- ^ "Monographs: Pharmaceutical substances: Metrifonate (Metrifonatum)". The International Pharmacopoeia Fourth Edition. WHO. Retrieved 2015-10-20.
- ^ "Helminths: Schistosomiasis: Metrifonate". WHO Model Prescribing Information: Drugs Used in Parasitic Diseases - Second Edition. WHO. 1995. Retrieved 2015-10-20.
- ^ Ross A.G.P.; Bartley P.B.; Sleigh A.C.; Olds G.R.; Li Y.; Williams G.M.; McManus D.P. (2002). "Schistosomiasis". The New England Journal of Medicine. 346 (16): 1212–1220. doi:10.1056/NEJMra012396. PMID 11961151.
- ^ López-Arrieta J, Schneider L (2008). López-Arrieta, Jess, ed. "Metrifonate for Alzheimer's disease". Cochrane Database of Systematic Reviews (1): CD003155. doi:10.1002/14651858.CD003155.pub3. PMID 16625573.
| This antiinfective drug article is a stub. You can help Wikipedia by expanding it. |