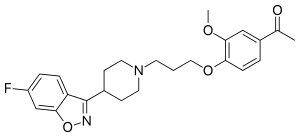
Iloperidone
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Fanapt, Zomaril |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a609026 |
| License data |
|
| Routes of administration |
Oral, injection |
| ATC code | N05AX14 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 96% (oral; Tmax = 2–4 hours) |
| Protein binding | ~97% |
| Metabolism | Hepatic (CYP2D6-mediated hydroxylation and CYP3A4-mediated O-demethylation) |
| Biological half-life | 18–33 hours |
| Excretion | urine (45.1–58.2%) and feces (19.9–22.1%) |
| Identifiers | |
|
|
| CAS Number | 133454-47-4 |
| PubChem (CID) | 71360 |
| IUPHAR/BPS | 87 |
| ChemSpider | 64459 |
| UNII | VPO7KJ050N |
| KEGG | D02666 |
| ChEBI | CHEBI:65173 |
| ChEMBL | CHEMBL14376 |
| ECHA InfoCard | 100.106.441 |
| Chemical and physical data | |
| Formula | C24H27FN2O4 |
| Molar mass | 426.481g/mol |
| 3D model (Jmol) | Interactive image |
|
|
|
|
| |
|
Iloperidone, also known as Fanapt, Fanapta, and previously known as Zomaril, is an atypical antipsychotic for the treatment of schizophrenia.
Contents
Availability[edit]
It was approved by the U.S. Food and Drug Administration (FDA) for use in the United States on May 6, 2009.
Pharmacology[edit]
Iloperidone is a monoamine directed towards acting upon and antagonizing specific neurotransmitters, particularly multiple dopamine and serotonin receptor subtypes. It is considered an ‘atypical’ antipsychotic because it displays serotonin receptor antagonism, similar to other atypical antipsychotics. The older typical antipsychotics are primarily dopamine antagonists.
Iloperidone has been shown to act as an antagonist at all tested receptors. It exhibits high (nM) affinity to serotonin 5HT2A (Ki value of 5.6 nM), dopamine D2 (6.3 nM) and D3 (7.1 nM) and noradrenaline α1 receptors (0.36 nM), moderate affinity for dopamine D4 (25 nM), serotonin 5HT6 (43 nM), 5HT7 (22 nM), and low affinity for the serotonin 5HT1A (168 nM), dopamine D1 and histamine H1 receptors. In addition, pharmacogenomic studies identified single nucleotide polymorphisms associated with an enhanced response to iloperidone during acute treatment of schizophrenia.[1][2]
Laboratory studies[edit]
Iloperidone performed well against a prepulse inhibition (PPI) experiment, which was designed to gauge the extent of sensory gating in rats, a process disrupted in schizophrenia. Prepulse inhibition is the reduction in the amount of startle the subject gives when presented with a non-startling stimulus. Those exhibiting high levels of psychosis present a deficit in PPI. Psychosis induced using PCP, apomorphine, and cirazoline, were all prevented with the concurrent administration of iloperidone. The PPI deficit normally incurred by each psychotic drug was significantly diminished by the co administration of iloperidone.[3] The results of this experiment provided strong evidence for iloperidone’s merit as an effective treatment for psychotic disorders. Iloperidone has also been shown to reduce the effects of apomorphine induced climbing behavior in mice as well as the effects of head twitching induced by 5-HT in rats.[4]
Clinical studies[edit]
Several large (n > 570 per trial), double-blind, multinational, multicentre trials showed iloperidone generally had better efficacy than placebo when using the Positive and Negative Syndrome Scale (PANSS) or Brief Psychiatric Rating Scale (BPRS) scores.[1] Clinical studies have also shown that some patients treated with iloperidone show reduced extrapyramidal symptoms and weight gain. Phase II testing has shown that effectiveness in humans is possible with as low as 8 mg per day, and is tolerable up to 4 mg per day.
Side effects[edit]
Examination of the safety and tolerability of iloperidone have shown that at a 5 mg/day dose in healthy male volunteers, the drug was fairly well tolerated, although hypotension, dizziness, and somnolence were very common side effects ranging from mild to moderate in severity. A second study showed that co administration of food decreased the severity of these effects. This study also indicated that repeat administration of iloperidone could decrease the effects of hypotension.[5]
The approved dose is 12–24 mg not 5 mg. However, claims of better tolerance have been reported.
In some cases (prevalence unknown), it can greatly increase agitation and aggressivity like all antipsychotics drugs.
Dosage[edit]
Vanda Pharmaceuticals has stated that they are developing both oral and injectable formulations. The injectable formulation is being developed to be administered at four week intervals.
Regulatory approval[edit]
Hoechst Marion Roussel Inc. made initial inquiries into the drug; however, in May 1996, they discontinued research, and in June 1997 gave research rights to Titan Pharmaceuticals. Titan then handed over worldwide development, manufacturing and marketing rights to Novartis in August 1998. On June 9, 2004, Titan Pharmaceuticals announced that the Phase III development rights have been acquired by Vanda Pharmaceuticals. The original launch date was scheduled for 2002. On November 27, 2007, Vanda Pharmaceuticals announced that the U.S. Food and Drug Administration (FDA) had accepted their New Drug Application for iloperidone, confirming the application is ready for FDA review and approval.[6] On July 28, 2008, the FDA issued a "Not Approvable" letter to Vanda Pharmaceuticals concerning the drug, stating that further trials are required before a decision can be made concerning marketed usage of iloperidone.[7]
Iloperidone won FDA approval for use treating schizophrenia in the United States on May 6, 2009.[8]
References[edit]
- ^ a b Scott L.Iloperidone: In Schizophrenia. CNSDrugs 2009; 23(10):867-880. doi:10.2165/10489070-000000000-00000.
- ^ Brunton, LL (2010). Goodman and Gilman's The Pharmacological Basis of Therapeutics, Twelfth Edition. New York, NY: McGraw-Hill Medical. ISBN 9780071769396.
- ^ Barr AM, Powell SB, Markou A, Geyer MA (September 2006). "Iloperidone reduces sensorimotor gating deficits in pharmacological models, but not a developmental model, of disrupted prepulse inhibition in rats". Neuropharmacology. 51 (3): 457–65. doi:10.1016/j.neuropharm.2006.04.004. PMID 16762376.
- ^ Szewczak MR, Corbett R, Rush DK, Wilmot CA, Conway PG, Strupczewski JT, Cornfeldt M (September 1995). "The pharmacological profile of iloperidone, a novel atypical antipsychotic agent". The Journal of Pharmacology and Experimental Therapeutics. 274 (3): 1404–13. PMID 7562515.
- ^ Sainati SM, Hubbard JW, Chi E, Grasing K, Brecher MB (July 1995). "Safety, tolerability, and effect of food on the pharmacokinetics of iloperidone (HP 873), a potential atypical antipsychotic". Journal of Clinical Pharmacology. 35 (7): 713–20. doi:10.1002/j.1552-4604.1995.tb04112.x. PMID 7560252.
- ^ "Vanda Pharmaceuticals Receives FDA Acceptance of Iloperidone New Drug Application" (Press release). Vanda Pharmaceuticals. November 27, 2007. Retrieved 2007-11-27.
- ^ "FDA Issues Not Approvable Letter for Iloperidone to Vanda Pharmaceuticals" (Press release). Vanda Pharmaceuticals. July 28, 2008. Retrieved 2008-08-08.
- ^ "Vanda's Schizophrenia Drug Wins Approval From U.S. Regulators" (Press release). Bloomberg. May 6, 2009. Retrieved 2009-05-06.