Formetorex
 |
|
| Names | |
|---|---|
| IUPAC name
N-(1-Phenylpropan-2-yl)formamide
|
|
| Other names
Formetorex
N-Formylamphetamine N-(alpha-Methylphenethyl)formamide |
|
| Identifiers | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL1395071 |
| ChemSpider | 59055 |
| PubChem | 65614 |
|
|
|
|
| Properties | |
| C10H13NO | |
| Molar mass | 163.22 g·mol−1 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |
Formetorex (INN), also known as formetamide or N-formylamphetamine, is an amphetamine described as an anorectic which does not appear to have ever been marketed.[1]
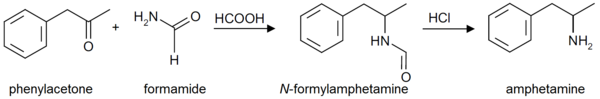
Formetorex is also an intermediate in the production of amphetamine by the "Leuckart reaction."[2] It is also commonly found as an impurity in clandestine labs where this synthesis method is used.[2][3] Due to the simplicity of the Leuckart reaction, it is the most popular synthetic route employed for the illicit manufacture of amphetamines.[2] The synthesis involves a non-metal reduction that is typically carried out in three steps.[2] For amphetamine synthesis, a mixture of phenylacetone and formamide (sometimes in the presence of formic acid) or ammonium formate, is heated until a condensation reaction results in the intermediate product, formetamide.[2] In the second step, formetamide is hydrolysed using hydrochloric acid, and the reaction mixture is then basified, isolated, and steam distilled to produce the free base.[2] The final step, the product is dissolved in an organic solvent and precipitated as the sulphate salt of amphetamine by adding sulfuric acid.[2]
References[edit]
- ^ C.R. Ganellin; David J. Triggle (21 November 1996). Dictionary of Pharmacological Agents. CRC Press. pp. 568–. ISBN 978-0-412-46630-4.
- ^ a b c d e f g Laboratory and Scientific Section (2006). Recommended methods of the identification and analysis of amphetamine, methamphetamine, and their ring-substituted analogues in seized materials (PDF). New York: United Nations Office on Drugs and Crime Vienna. ISBN 92-1-148208-9. Retrieved 14 October 2013.
- ^ Kram, TC (July 1979). "Reidentification of a major impurity in illicit amphetamine.". Journal of forensic sciences. 24 (3): 596–9. PMID 541629.
External links[edit]
- formetamide at the US National Library of Medicine Medical Subject Headings (MeSH)
| This drug article relating to the nervous system is a stub. You can help Wikipedia by expanding it. |