Lofendazam
From Wikipedia, the free encyclopedia
 |
|
| Systematic (IUPAC) name | |
|---|---|
|
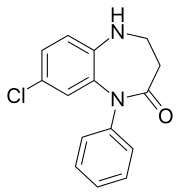
8-chloro-1-phenyl-1,3,4,5-tetrahydro- 2H-1,5-benzodiazepin- 2-one
|
|
| Identifiers | |
| CAS Number | 29176-29-2 |
| ATC code | none |
| PubChem | CID 71709 |
| ChemSpider | 64759 |
| UNII | V7O53S50SN |
| Chemical data | |
| Formula | C15H13ClN2O |
| Molar mass | 272.73 |
| 3D model (Jmol) | Interactive image |
|
|
|
|
| (verify) | |
Lofendazam[1] is an organic molecule which is a benzodiazepine derivative. Lofendazam is a 1,5-benzodiazepine, with the nitrogen atoms located at positions 1 and 5 of the diazepine ring; therefore, lofendazam is most closely related to other 1,5-benzodiazepines such as clobazam. [2][3]
Lofendazam as a human pharmaceutical has sedative and anxiolytic effects similar to those produced by other benzodiazepine derivatives. It is an active metabolite of another benzodiazepine, arfendazam.[4]
See also[edit]
References[edit]
- ^ DE Patent 1929656
- ^ Malik F, Hasan M, Khan KM, Perveen S, Snatzke G, Duddeck H, Voelter W. Syntheses and CD studies of optically active substituted 1,3,4,5-tetrahydro- 2H-1,5-benzodiazepin- 2-ones. Liebigs Annalen der Chemie, 1995. (10):1861-1869.
- ^ Aversa MC, Giannetto P, Romeo G, Ficarra P, Vigorita MG. Nuclear magnetic resonance spectra of psychotherapeutic agents. V* - conformational analysis of 1,3,4,5-tetrahydro- 2H-1,5-benzodiazepin- 2-ones. Organic Magnetic Resonance, 1981. 15(4):394-398
- ^ J. Adrien, F. Albani, A. Baruzzi, M. Berger, E.O. Bixler, A.A. Borbeley, D.G. Dikeos, R. Drucker-Colin, R. Fritsch Montero, Y. Hishikawa, S. Inoue, A. Kales, E. Lugaresi, H. Merchant-Nancy, J.M. Monti. The Pharmacology of Sleep. Springer. ISBN 978-3-540-58961-7