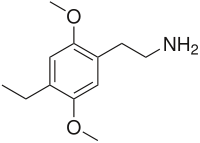
2C-E
|
|
This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these template messages)
(Learn how and when to remove this template message)
|
 |
|||
|
|
|||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
4-Ethyl-2,5-dimethoxyphenethylamine
|
|||
| Other names
2,5-Dimethoxyphenethylamine, Aquarust
|
|||
| Identifiers | |||
| 71539-34-9 |
|||
| 3D model (Jmol) | Interactive image | ||
| ChEMBL | ChEMBL124063 |
||
| ChemSpider | 21106222 |
||
| ECHA InfoCard | 100.221.016 | ||
| PubChem | 24729233 | ||
| UNII | I190284UXX |
||
|
|||
|
|||
| Properties | |||
| C12H19NO2 | |||
| Molar mass | 209.2859 g/mol | ||
| Appearance | white crystals | ||
| >70mg/ml (20°C) | |||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
| Infobox references | |||
2C-E is a psychedelic phenethylamine of the 2C family. It was first synthesized by Alexander Shulgin.[1]
Shulgin classified 2C-E as a member of the "Magical Half-Dozen" in his book PiHKAL: A Chemical Love Story. Vivid visuals similar to those experienced while under the influence of LSD are common, and many reports indicate that the effects of this particular chemical may be overly intense for those not well experienced with psychedelics.[2]
Contents
Properties[edit]
2,5-Dimethoxy-4-ethylphenethylamine is a colorless oil. Crystalline forms are obtained as the amine salt by reacting the free base with a mineral acid, typically HCl.
Shulgin does not report an exact boiling point for the free base, stating only that during one synthesis the fraction boiling between 90–100 °C at 0.25 mmHg pressure was collected and converted to the hydrochloride salt. Shulgin reports the melting point of the hydrochloride salt as 208.5–210.5 °C.[3]
Effects[edit]
The total duration of 2C-E's effects is generally between six and ten hours for an average dose, with the plateau lasting between three and six hours.[4] For such a dose, the onset of effects takes approximately twenty to ninety minutes and perception may be somewhat altered for up to a day after ingestion.[5]
2C-E's effects are often described as "neutral", in comparison with other psychedelic chemicals and even other 2C-x related molecules. In PiHKAL, Shulgin states:
- "Here is another of the magical half-dozen. The range is purposefully broad. At 10 milligrams there have been some pretty rich +++[nb 1] experiences, and yet I have had the report from one young lady of a 30 milligram trial that was very frightening. My first experience with 2C-E was really profound, and it is the substance of a chapter within the story. Several people have said, about 2C-E, "I don't think I like it, since it isn't that much fun. But I intend to explore it again." There is something here that will reward the experimenter. Someday, the full character of 2C-E will be understood, but for the moment, let it rest as being a difficult and worth-while material. A very much worth-while material."
Adverse effects[edit]
At least one death has been attributed to a 2C-E overdose.[6]
Drug prohibition laws[edit]
Australia[edit]
In Queensland, 2C-E was added to the 'Dangerous Drugs' list of the 'Drugs Misuse Act 1986'[7] by the 'Drugs Misuse Amendment Act 2008'.[8] Making it illegal to produce, supply or possess.
Canada[edit]
As of October 31st, 2016; 2C-E is a controlled substance (Schedule III) in Canada. http://gazette.gc.ca/rp-pr/p2/2016/2016-05-04/html/sor-dors72-eng.php
China[edit]
As of October 2015 2C-E is a controlled substance in China.[9]
Denmark[edit]
2C-E is added to the list of Schedule B controlled substances.[10]
Germany[edit]
2C-E is a Anlage I controlled drug.
New Zealand[edit]
New Zealand has a catch-all Analogues section in Schedule 3 / Class C of their drug laws that would make 2C-I, 2C-E, DOI, ephedrine, and pseudoephedrine Schedule 3 compounds in New Zealand.
Sweden[edit]
Sveriges riksdags health ministry Statens folkhälsoinstitut classified 2C-E as "health hazard" under the act Lagen om förbud mot vissa hälsofarliga varor (translated Act on the Prohibition of Certain Goods Dangerous to Health) as of Oct 1, 2004, in their regulation SFS 2004:696 listed as 2,5-dimetoxi-4-etylfenetylamin (2C-E), making it illegal to sell or possess.[11]
UK[edit]
In the United Kingdom, 2C-E is a Class A controlled substance. The UK has the strictest laws in the EU on designer drugs. The Misuse Of Drugs Act was amended in 2002 to include a "catch most" clause outlawing every drug, and possible future drug, from the LSD (ergoline) and MDMA (phenethylamine) chemical families (including 2C-E). The amendment is a near verbatim quote from the books of the American biochemist Alexander Shulgin, who obtained a PhD from the University of California, Berkeley. Dr. Shulgin, a former research chemist at the Dow Chemical Company, re-discovered the synthesis for MDMA in 1976 and published the syntheses for more than 200 phenethylamine compounds of his own invention, and 55 tryptamine compounds many of which were also his own invention. The Shulgins were motivated to release the synthesis information as a way to protect the public's access to information about psychedelic compounds, a goal Alexander Shulgin has noted many times.
USA[edit]
As of July 9, 2012, in the United States 2C-E is a Schedule I substance under the Food and Drug Administration Safety and Innovation Act of 2012, making possession, distribution and manufacture illegal.[12]
Notes[edit]
- ^ Shulgin's +/- rating scale, per PiHKAL. See References below. Quoting: "Plus Three (+++) = Not only are the chronology and the nature of a drug's action quite clear, but ignoring its action is no longer an option. The subject is totally engaged in the experience, for better or worse."
References[edit]
- ^ Erowid
- ^ Shulgin, Alexander; Ann Shulgin (September 1991). PiHKAL: A Chemical Love Story. Berkeley, California: Transform Press. ISBN 0-9630096-0-5. OCLC 25627628.
- ^ Shulgin, Alexander T.; Manning, Tania; Daley, Paul F. (2011). The Shulgin Index: Volume 1 (First ed.). Berkeley, CA: Transform Press. ISBN 978-0-9630096-3-0.
- ^ Shulgin, Alexander T; Shulgin, Ann (1991). PiHKAL: A Chemical Love Story (1st ed., 7th printing ed.). Berkeley, CA: Transform Press. ISBN 978-0-9630096-0-9.
- ^ Fire, Erowid; Earth, Erowid. "Erowid 2C-E Vault: Effects". Erowid.org. Retrieved 29 June 2014.
- ^ Pham, Sherisse (18 March 2011). "Man Arrested in Mass Drug Overdose That Killed 1 Teen and Left 10 People Hospitalized". ABC World News. Retrieved 29 June 2014.
- ^ https://www.legislation.qld.gov.au/LEGISLTN/CURRENT/D/DrugsMisuseA86.pdf
- ^ https://www.legislation.qld.gov.au/LEGISLTN/ACTS/2008/08AC004.pdf
- ^ "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in Chinese). China Food and Drug Administration. 27 September 2015. Retrieved 1 October 2015.
- ^ Bekendtgørelse om euforiserende stoffer - retsinformation.dk
- ^ http://www.notisum.se/rnp/sls/sfs/20040696.pdf
- ^ Erowid.org, Legal Status of 2C-E




