Quadrosilan
From Wikipedia, the free encyclopedia
 |
|
| Identifiers | |
|---|---|
|
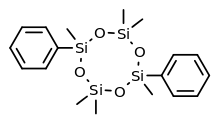
Systematic (IUPAC) name: 2,2,4,6,6,8-Hexamethyl-4,8-diphenyl-1,3,5,7,2,4,6,8-tetraoxatetrasilocane
|
|
| Synonyms | Quadrosilane |
| CAS Number | 4657-20-9 |
| PubChem (CID) | 20774 |
| ChemSpider | 19557 |
| Chemical and physical data | |
| Formula | C18H28O4Si4 |
| Molar mass | 420.106466 g/mol |
| 3D model (Jmol) | Interactive image |
|
|
|
|
Quadrosilan (INN, BAN) (trade name Cisobitan; former developmental code name KABI-1774) is a synthetic, non-steroidal estrogen that was developed in the 1970s and that is or has been used as an antigonadotropic agent in the treatment of prostate cancer.[1][2][3][4] It is an organosilicon compound, and is also known as 2,6-cisdiphenylhexamethylcyclotetrasiloxane.[3][5] Quadrosilan has estrogenic activity equivalent to that of estradiol,[6] and can produce feminization and gynecomastia as side effects in male patients.[7][8]
See also[edit]
References[edit]
- ^ J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 629–. ISBN 978-1-4757-2085-3.
- ^ I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 243–. ISBN 978-94-011-4439-1.
- ^ a b Alfthan, Olof; Andersson, Lennart; Luigi Esposti, Pier; Dorothea Fosså, Sophie; Arne Gammelgaard, Peter; Erik Gjöres, Jan; Isacson, Sune; Rasmussen, Finn; Ruutu, Mirja; von Schreeb, Tor; Setterberg, Gun; Strandell, Per; Strindberg, Bengt (1983). "Cisobitan® in Treatment of Prostatic Cancer". Scandinavian Journal of Urology and Nephrology. 17 (1): 37–43. doi:10.3109/00365598309179778. ISSN 0036-5599.
- ^ Chisholm, Geoffrey D. (1985). "Treatment of advanced cancer of the prostate". Seminars in Surgical Oncology. 1 (1): 38–55. doi:10.1002/ssu.2980010106. ISSN 8756-0437.
- ^ Yitzhak Apeloig (1989). The Chemistry of Organic Silicon Compounds. John Wiley & Sons Canada, Limited. p. 1154. ISBN 978-0-471-91993-3.
- ^ Mills, John S; Showell, Graham A (2004). "Exploitation of silicon medicinal chemistry in drug discovery". Expert Opinion on Investigational Drugs. 13 (9): 1149–1157. doi:10.1517/13543784.13.9.1149. ISSN 1354-3784.
- ^ Strindberg, Bengt (1978). "Biochemical Effects of 2, 6-cis-Diphenylhexamethylcyclotetrasiloxane in Man": 515–520. doi:10.1007/978-1-4613-4018-8_23.
- ^ Krarup T, Rasmussen F, Gammelgaard PA (1978). "Prostatic carcinoma treated with 2,6-cis-diphenylhexamethylcyclotetrasiloxane (Cisobitan)". Scand. J. Urol. Nephrol. 12 (1): 11–5. PMID 345431.