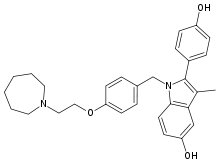
Bazedoxifene
 |
|
 |
|
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| License data | |
| Routes of administration |
Oral |
| ATC code | G03XC02 (WHO) |
| Legal status | |
| Legal status |
|
| Identifiers | |
|
Systematic (IUPAC) name: 1-{4-[2-(Azepan-1-yl)ethoxy]benzyl}-2-(4-hydroxyphenyl)-3-methyl-1H-indol-5-ol
|
|
| CAS Number | 198481-32-2 |
| PubChem (CID) | 154257 |
| IUPHAR/BPS | 7355 |
| DrugBank | DB06401 |
| ChemSpider | 135921 |
| UNII | Q16TT9C5BK |
| ChEMBL | CHEMBL46740 |
| PDB ligand ID | 29S (PDBe, RCSB PDB) |
| Chemical and physical data | |
| Formula | C30H34N2O3 |
| Molar mass | 470.603 g/mol |
| 3D model (Jmol) | Interactive image |
|
|
|
|
| |
|
Bazedoxifene, or bazedoxifene acetate, is a third generation selective estrogen receptor modulator (SERM), developed by Pfizer following the completion of their takeover of Wyeth Pharmaceuticals. In late 2013, Pfizer received approval for bazedoxifene as part of the combination drug DUAVEE in the prevention (not treatment) of postmenopausal osteoporosis.[1] Bazedoxfiene is the result of an exclusive research collaboration between Wyeth Pharmaceuticals and Ligand Pharmaceuticals.
It is approved in the European Union (marketed in Italy and Spain) and Japan, and is in the late phases of review by the United States' Food and Drug Administration (FDA). When approved, bazedoxifene is to be sold by Pfizer under the tradename Viviant in the US, Japan and Conbriza in the EU. Bazedoxifene's combination with conjugated estrogens, Aprela, is undergoing Phase III studies for the treatment of postmenopausal symptoms (including the prevention of postmenopausal osteoporosis/treatment of osteopenia).
The drug is being investigated for possible use in dyspareunia (painful sexual intercourse).
It is also being studied for possible treatment of breast cancer and pancreatic cancer.[2]
History of approval[edit]
Wyeth received an approvable letter for bazedoxifene in late April 2007. The FDA called for final safety and efficacy data from Phase III studies, and acceptable valuation of manufacturing and testing facilities where problems were found earlier in the year. Wyeth was working with the FDA to resolve these issues, and expected an FDA action date at the end of 2007.
As of December 2011, the FDA have not given approval for the use of bazedoxifene in the US.[3]
The drug was approved in the European Union by the European Medicines Agency on April 27, 2009.[4]
On October 3, 2013 the FDA approved the combination product of bazedoxifene 20 mg with 0.45 mg Premarin (conjugated estrogens) for the treatment of menopausal osteoporosis and the treatment of moderate to severe hot flushes. This is the first approved hormone replacement therapy product that contains a SERM (bazedoxifene) and an estrogen. The result is a hormone replacement therapy product for women who have an intact uterus that does not require a progestin. As progestins are associated with many of the tolerability and safety issues ascribed to hormone replacement therapy, there are several potential advantages to a progestin free regimen. Unlike premarin combinations with the progestin medroxyprogesterone acetate ("MPA"), phase 3 clinical studies with bazedoxifene/premarin did not increase vaginal bleeding, breast tenderness or breast density. In a direct comparison between bazedoxifene/premarin with MPA/premarin, there were significantly less adverse events in the bazedoxifene/premarin groups. The lack of breast density increases and breast pain with the combination therapy suggest that unlike estrogen combinations with progestin, this therapy could have a more neutral or even beneficial profile on the breast. The combination is expected to launch in the US in the first quarter of 2014 under the trade name DUAVEE. The drug application for the combination is pending in the EU, where bazedoxifene is already approved as a monotherapy for osteoporosis.
Duavee is the new drug, alternative to Prempro for women who want to use estrogen for menopausal symptoms but need an alternative to a progestin.
See also[edit]
References[edit]
- ^ Biskobing, D. M. (2007). "Update on bazedoxifene: A novel selective estrogen receptor modulator". Clinical interventions in aging. 2 (3): 299–303. PMC 2685267
 . PMID 18044180.
. PMID 18044180. - ^ http://medicalxpress.com/news/2013-06-osteoporosis-drug-growth-breast-cancer.html
- ^ "Drugs@FDA - Drug Names Beginning with "B"". Food and Drug Administration. 7 July 2009. Archived from the original on 11 July 2009. Retrieved 2009-07-08.
- ^ "EPARs for authorised medicinal products for human use - Conbriza". European Medicines Agency. 26 May 2009. Archived from the original on 11 June 2009. Retrieved 2009-07-08.