Trimebutine
From Wikipedia, the free encyclopedia
 |
|
| Systematic (IUPAC) name | |
|---|---|
|
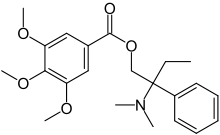
2-(Dimethylamino)-2-phenylbutyl 3,4,5-trimethoxybenzoate
|
|
| Clinical data | |
| AHFS/Drugs.com | International Drug Names |
| Identifiers | |
| CAS Number | 39133-31-8 |
| ATC code | A03AA05 (WHO) |
| PubChem | CID 5573 |
| ChemSpider | 5372 |
| UNII | QZ1OJ92E5R |
| ChEMBL | CHEMBL190044 |
| Chemical data | |
| Formula | C22H29NO5 |
| Molar mass | 387.47 g/mol |
| 3D model (Jmol) | Interactive image |
|
|
|
|
| |
|
Trimebutine is a drug with antimuscarinic and weak mu opioid agonist effects.[1] The maleic acid salt of trimebutine is marketed under the trademark of Debridat, Recutin, Polybutin,[2] or Modulon for treatment of irritable bowel syndrome and other gastrointestinal disorders. The major product from drug metabolism of trimebutine in human beings is nortrimebutine,[3] which comes from removal of one of the methyl groups attached to nitrogen. Trimebutine exerts its effects in part due to causing a premature activation of phase III of the migrating motor complex in the digestive tract.[4] Both Trimebutine and its metabolite are commercially available.
See also[edit]
References[edit]
- ^ Kaneto H, Takahashi M, Watanabe J. The opioid receptor selectivity for trimebutine in isolated tissues experiments and receptor binding studies. Journal of Pharmacobiodynamics. 1990 Jul;13(7):448-53. PMID 1963196
- ^ Ok Hwa Jhee, Yun Sik Lee, Leslie M. Shaw, Yong Cheol Jeon, Min Ho Lee, Seung Hoon Lee and Ju Seop Kang The Pharmacokinetic and bioequivalence evaluation of two formulations of 100 mg trimebutine maleate (Recutin and Polybutin) in healthy male volunteers using the LC–MS/MS method. Clinica Chimica Acta. 2007 Jan;375(1-2):69-75. PMID 16854404
- ^ F.J. Roman, S. Lanet, J. Hamon, G. Brunelle, A. Maurin, P. Champeroux, S. Richard, N. Alessandri, and M. Gola Pharmacological Properties of Trimebutine and N-Monodesmethyltrimebutine. The Journal of Pharmacology and Experimental Therapeutics 1999;289:1391–1397. PMID 10336531
- ^ Hiyama, T.; Yoshihara, M.; Tanaka, S.; Haruma, K.; Chayama, K. (Apr 2009). "Effectiveness of prokinetic agents against diseases external to the gastrointestinal tract." (PDF). J Gastroenterol Hepatol. 24 (4): 537–46. doi:10.1111/j.1440-1746.2009.05780.x. PMID 19220673.
| This drug article relating to the gastrointestinal system is a stub. You can help Wikipedia by expanding it. |