Pregnanolone
From Wikipedia, the free encyclopedia
(Redirected from Eltanolone)
Jump to: navigation, search
 |
|
| Names | |
|---|---|
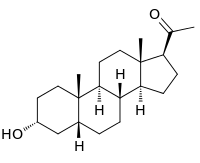
| IUPAC name
(3α,5β)-3-Hydroxypregnan-20-one
|
|
| Other names
Eltanolone; 5β-Pregnan-3α-ol-20-one; 3α-Hydroxy-5β-pregnan-20-one; 3α-Hydroxy-5β-tetrahydroprogesterone
|
|
| Identifiers | |
| 128-20-1 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:1712 |
| ChEMBL | ChEMBL210952 |
| ChemSpider | 29132 |
| ECHA InfoCard | 100.162.192 |
| PubChem | 31402 |
|
|
|
|
| Properties | |
| C21H34O2 | |
| Molar mass | 318.50 g·mol−1 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |
This article is about the 3α,5β-reduced metabolite of progesterone. For other pregnanolone isomers of progesterone, see Pregnanolone (disambiguation).
Pregnanolone, also known as eltanolone (INN), as well as 3α,5β-tetrahydroprogesterone (3α,5β-THP) or 3α-hydroxy-5β-pregnan-20-one, is an endogenous neurosteroid that is biosynthesized from progesterone.[1] It is a positive allosteric modulator of the GABAA receptor,[1] as well as a negative allosteric modulator of the glycine receptor,[2] and is known to have sedative, anxiolytic, anesthetic, and anticonvulsant effects.[1][2][3] It was investigated for clinical use as a general anesthetic, but produced unwanted side effects such as convulsions on occasion, and for that reason was never marketed.[2][4]
See also[edit]
References[edit]
- ^ a b c Reddy DS (2003). "Pharmacology of endogenous neuroactive steroids". Crit Rev Neurobiol. 15 (3-4): 197–234. doi:10.1615/critrevneurobiol.v15.i34.20. PMID 15248811.
- ^ a b c Jürgen Schüttler; Helmut Schwilden (8 January 2008). Modern Anesthetics. Springer Science & Business Media. pp. 278–. ISBN 978-3-540-74806-9.
- ^ Carl P, Høgskilde S, Lang-Jensen T, et al. (October 1994). "Pharmacokinetics and pharmacodynamics of eltanolone (pregnanolone), a new steroid intravenous anaesthetic, in humans". Acta Anaesthesiol Scand. 38 (7): 734–41. doi:10.1111/j.1399-6576.1994.tb03987.x. PMID 7839787.
- ^ Norman Calvey; Norton Williams (21 January 2009). Principles and Practice of Pharmacology for Anaesthetists. John Wiley & Sons. pp. 110–. ISBN 978-1-4051-9484-6.
| Alcohols | |
|---|---|
| Barbiturates |
|
| Benzodiazepines |
|
| Carbamates | |
| Flavonoids |
|
| Imidazoles | |
| Kava constituents | |
| Monoureides | |
| Neuroactive steroids |
|
| Nonbenzodiazepines |
|
| Phenols | |
| Piperidinediones | |
| Pyrazolopyridines | |
| Quinazolinones | |
| Volatiles/gases |
|
| Others/unsorted |
|
|
See also: GABAergics
|
|
| Receptor (ligands) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transporter (blockers) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
| Enzyme (inhibitors) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
| Others |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
| Receptor (ligands) |
|
||||||
|---|---|---|---|---|---|---|---|
| Transporter (blockers) |
|
||||||
| Others |
|
||||||
| CAR |
|
||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ERR |
|
||||||||||||||||||||||||||||||||||||||||||||||||
| FXR |
|
||||||||||||||||||||||||||||||||||||||||||||||||
| LXR |
|
||||||||||||||||||||||||||||||||||||||||||||||||
| PPAR |
|
||||||||||||||||||||||||||||||||||||||||||||||||
| PXR |
|
||||||||||||||||||||||||||||||||||||||||||||||||
| RAR |
|
||||||||||||||||||||||||||||||||||||||||||||||||
| RXR |
|
||||||||||||||||||||||||||||||||||||||||||||||||
| SHR |
|
||||||||||||||||||||||||||||||||||||||||||||||||
| TR |
|
||||||||||||||||||||||||||||||||||||||||||||||||
| This article about a steroid is a stub. You can help Wikipedia by expanding it. |
| This drug article relating to the nervous system is a stub. You can help Wikipedia by expanding it. |