Camazepam
 |
|
 |
|
| Systematic (IUPAC) name | |
|---|---|
|
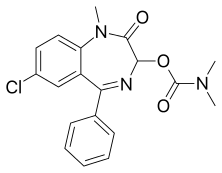
7-chloro-1-methyl-2-oxo-5-phenyl-2,3-dihydro-1H-1,4-benzodiazepin-3-yl N,N-dimethylcarbamate
|
|
| Clinical data | |
| Pregnancy category |
|
| Routes of administration |
Oral |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 90% |
| Metabolism | Hepatic |
| Biological half-life | 6,4-10,5 hours |
| Excretion | Renal |
| Identifiers | |
| CAS Number | 36104-80-0 |
| ATC code | N05BA15 (WHO) |
| PubChem | CID 37367 |
| DrugBank | DB01489 |
| ChemSpider | 34285 |
| UNII | HZ3XRH03C3 |
| KEGG | D07315 |
| ChEMBL | CHEMBL1095282 |
| Chemical data | |
| Formula | C19H18ClN3O3 |
| Molar mass | 371.8 |
| 3D model (Jmol) | Interactive image |
|
|
|
|
| (verify) | |
Camazepam[1] is a benzodiazepine psychoactive drug, marketed under the brand names Albego, Limpidon and Paxor. It is the dimethyl carbamate ester of temazepam, a metabolite of diazepam.[2] While it possesses anxiolytic, anticonvulsant, skeletal muscle relaxant and hypnotic properties[3] it differs from other benzodiazepines in that its anxiolytic properties are particularly prominent but has comparatively limited anticonvulsant, hypnotic and skeletal muscle relaxant properties.
Contents
Pharmacology[edit]
Camazepam, like others benzodiazepines, produce a variety of therapeutic and adverse effects by binding to the benzodiazepine receptor site on the GABAA receptor and modulating the function of the GABA receptor, the most prolific inhibitory receptor within the brain. The GABA chemical and receptor system mediates inhibitory or calming effects of camazepam on the nervous system. Compared to other benzodiazepines, it has reduced side effects such as impaired cognition, reaction times and coordination.[4][5][6][7][8] which makes it best suited as an anxiolytic because of these reduced sides effects. Animal studies have shown camazepam and its active metabolites possess anticonvulsant properties.[9] Unlike other benzodiazepines it does not disrupt normal sleep patterns.[10] Camazepam has been shown in animal experiments to have a very low affinity for benzodiazepine receptors compared to other benzodiazepines.[11] Compared to temazepam, camazepam has shown roughly equal anxiolytic properties, and less anticonvulsant, sedative and motor-impairing properties.
Pharmacokinetics[edit]
Following oral administration, camazepam is almost completely absorbed into the bloodstream, with 90 percent bioavailability achieved in humans.[12] In the human camazepam is metabolised into the active metabolite temazepam.[13] Studies in dogs have shown that the half-life of the terminal elimination phase ranged from 6.4 to 10.5 h.[14]
Medical uses[edit]
Camazepam is indicated for the short-term treatment of insomnia and anxiety. As with other benzodiazepines, its use should be reserved for those patients in which the sleep disorder is severe, disabling or causes marked distress.
Adverse effects[edit]
With higher doses such as 40 mg of camazepam, impairments similar to those caused by other benzodiazepines manifest as disrupted sleep patterns and impaired cognitive performance.[15] Skin disorders have been reported with use of camazepam however.[16] One study has shown that camazepam may increase attention.[17]
Contraindications[edit]
Use of camazepam is contraindicated in subjects with known hypersensitivity to drug or allergy to other drugs in the benzodiazepine class or any excipients contained in the pharmaceutical form. Use of camazepam should be avoided or carefully monitored by medical professionals in individuals with the following conditions: myasthenia gravis, severe liver deficiencies (e.g., cirrhosis), severe sleep apnea, pre-existing respiratory depression or cronic pulmonary insufficiency.
See also[edit]
References[edit]
- ^ DE Patent 2142181
- ^ Tammaro, A; Picceo, MT; Gemmellaro, P; Bonaccorso, O (1977). "Camazepam versus placebo. A double-blind clinical study on geriatric patients suffering from psychic complaints. Short Communication". Arzneimittel-Forschung. 27 (11): 2177–8. PMID 23793.
- ^ Lu, XL; Yang, SK (1994). "Enantiomer resolution of camazepam and its derivatives and enantioselective metabolism of camazepam by human liver microsomes.". Journal of Chromatography A. 666 (1–2): 249–57. doi:10.1016/0021-9673(94)80387-0. PMID 7911374.
- ^ De Sarro, G; Chimirri, A; Zappala, M; Guisti, P; Lipartiti, M; De Sarro, A (1996). "Azirino1, 2-d1, 4benzodiazepine derivatives and related 1,4-benzodiazepines as anticonvulsant agents in DBA/2 mice". General Pharmacology. 27 (7): 1155–62. doi:10.1016/S0306-3623(96)00049-3. PMID 8981061.
- ^ De Sarro, G; Gitto, R; Rizzo, M; Zappia, M; De Sarro, A (1996). "1,4-Benzodiazepine derivatives as anticonvulsant agents in DBA/2 mice". General Pharmacology. 27 (6): 935–41. doi:10.1016/0306-3623(95)02147-7. PMID 8909973.
- ^ De Sarro, G; Chimirri, A; Mckernan, R; Quirk, K; Giusti, P; De Sarro, A (1997). "Anticonvulsant activity of azirino1,2-d1,4benzodiazepines and related 1,4-benzodiazepines in mice". Pharmacology, Biochemistry, and Behavior. 58 (1): 281–9. doi:10.1016/S0091-3057(96)00565-5. PMID 9264104.
- ^ Guthy, H (1975). "The medicinal treatment of anxiety in alcoholism in the withdrawal stage (author's transl)". Munchener Medizinische Wochenschrift. 117 (35): 1387–90. PMID 241014.
- ^ Tallone, G; Ghirardi, P; Bianchi, MC; Ravaccia, F; Bruni, G; Loreti, P (1980). "Reaction time to acoustic or visual stimuli after administration of camazepam and diazepam in man". Arzneimittel-Forschung. 30 (6): 1021–4. PMID 6106497.
- ^ Morino, A; Sasaki, H; Mukai, H; Sugiyama, M (1986). "Receptor-mediated model relating anticonvulsant effect to brain levels of camazepam in the presence of its active metabolites". Journal of Pharmacokinetics and Biopharmaceutics. 14 (3): 309–21. doi:10.1007/BF01106709. PMID 2878071.
- ^ Ferrillo, F; Balestra, V; Carta, F; Nuvoli, G; Pintus, C; Rosadini, G (1984). "Comparison between the central effects of camazepam and temazepam. Computerized analysis of sleep recordings". Neuropsychobiology. 11 (1): 72–6. doi:10.1159/000118055. PMID 6146112.
- ^ Shibuya, T; Field, R; Watanabe, Y; Sato, K; Salafsky, B (1984). "Structure-affinity relationships between several new benzodiazepine derivatives and 3H-diazepam receptor sites". Japanese Journal of Pharmacology. 34 (4): 435–40. doi:10.1254/jjp.34.435. PMID 6144807.
- ^ Morino, A; Nakamura, A; Nakanishi, K; Tatewaki, N; Sugiyama, M (1985). "Species differences in the disposition and metabolism of camazepam". Xenobiotica. 15 (12): 1033–43. doi:10.3109/00498258509049098. PMID 2868575.
- ^ Riva, R; Albani, F; Baruzzi, A (1982). "Quantitative determination of camazepam and its metabolite temazepam in man by gas-liquid chromatography with electron-capture detection". Il Farmaco; edizione pratica. 37 (1): 15–9. PMID 6120096.
- ^ Legheand J, Cuisinaud G, Bernard N, Riotte M, Sassard J (1982). "Pharmacokinetics of intravenous camazepam in dogs". Arzneimittel-Forschung. 32 (7): 752–6. PMID 6127087.
- ^ Nicholson, AN; Stone, BM (1982). "Hypnotic activity and effects on performance of lormetazepam and camazepam--analogues of temazepam". British Journal of Clinical Pharmacology. 13 (3): 433–9. doi:10.1111/j.1365-2125.1982.tb01398.x. PMC 1402107
 . PMID 6120717.
. PMID 6120717. - ^ Stricker, BH (1984). "Skin disorders caused by the use of camazepam (Albego)". Nederlands tijdschrift voor geneeskunde. 128 (18): 870–2. PMID 6145108.
- ^ Schmitt, EJ; Rochels, R; Beck, D; Mauersberg, L (1980). "Visual perception under the influence of a tranquilizer (author's transl)". Klinische Monatsblätter für Augenheilkunde. 177 (6): 875–7. doi:10.1055/s-2008-1057748. PMID 6110803.