Wogonin
 |
|
 |
|
| Names | |
|---|---|
| IUPAC name
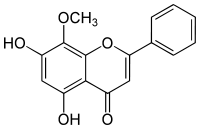
5,7-Dihydroxy-8-methoxy-2-phenyl-4H-chromen-4-one
|
|
| Other names | |
| Identifiers | |
| 632-85-9 |
|
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:10043 |
| ChEMBL | ChEMBL16171 |
| ChemSpider | 4445020 |
| KEGG | C10197 |
| PubChem | 5281703 |
|
|
|
|
| Properties | |
| C16H12O5 | |
| Molar mass | 284.27 g·mol−1 |
| Melting point | 203 to 206 °C (397 to 403 °F; 476 to 479 K) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |
Wogonin is an O-methylated flavone, a flavonoid-like chemical compound which was found in Scutellaria baicalensis.[2]
The glycosides of wogonin are known as wogonosides. For example, oroxindin is a wogonin glucuronide isolated from Oroxylum indicum.[3] It is one of the active ingredients of Sho-Saiko-To, a Japanese herbal supplement.
Wogonin has been found in one study to have anxiolytic properties in mice at doses of 7.5 to 30 mg/kg, without exhibiting the sedative and muscle-relaxing properties of benzodiazepines.[2] Preliminary in vitro studies have shown pharmacological effects that indicate wogonin may have anti-tumor properties.[4][5] Wogonin has also been found to possess anticonvulsant effects.[6] It acts as a positive allosteric modulator of the benzodiazepine site of the GABAA receptor.[2][6]
References[edit]
- ^ Wogonin at chemblink.com
- ^ a b c Hui KM, Huen MS, Wang HY, Zheng H, Sigel E, Baur R, Ren H, Li ZW, Wong JT, Xue H (2002). "Anxiolytic effect of wogonin, a benzodiazepine receptor ligand isolated from Scutellaria baicalensis Georgi". Biochem. Pharmacol. 64 (9): 1415–24. doi:10.1016/s0006-2952(02)01347-3. PMID 12392823.
- ^ Ramachandran Nair AG; Joshi BS. "Oroxindin—A new flavone glucuronide from Oroxylum indicum". doi:10.1007/BF02844710.
- ^ Lin CC, Kuo CL, Lee MH, Lai KC, Lin JP, Yang JS, Yu CS, Lu CC, Chiang JH, Chueh FS, Chung JG (2011). "Wogonin triggers apoptosis in human osteosarcoma U-2 OS cells through the endoplasmic reticulum stress, mitochondrial dysfunction and caspase-3-dependent signaling pathways". Int J Oncol. 39 (1): 217–224. doi:10.3892/ijo.2011.1027. PMID 21573491.
- ^ Gao J, Morgan WA, Sanchez-Medina A, Corcoran O (2011). "The ethanol extract of Scutellaria baicalensis and the active compounds induce cell cycle arrest and apoptosis including upregulation of p53 and Bax in human lung cancer cells". Toxicol Appl Pharmacol. 254 (3): 221–8. doi:10.1016/j.taap.2011.03.016. PMID 21457722.
- ^ a b Park HG, Yoon SY, Choi JY, Lee GS, Choi JH, Shin CY, Son KH, Lee YS, Kim WK, Ryu JH, Ko KH, Cheong JH (2007). "Anticonvulsant effect of wogonin isolated from Scutellaria baicalensis". Eur. J. Pharmacol. 574 (2-3): 112–9. doi:10.1016/j.ejphar.2007.07.011. PMID 17692312.
| This anticonvulsant-related article is a stub. You can help Wikipedia by expanding it. |