Trimethadione
From Wikipedia, the free encyclopedia
This article is about the drug Tridione. For the content management software Tridion, see SDL plc.
 |
|
| Clinical data | |
|---|---|
| Trade names | Tridione |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Pregnancy category |
|
| Routes of administration |
By mouth |
| ATC code | N03AC02 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | High |
| Metabolism | Demethylated to dimethadione |
| Biological half-life | 12–24 hours (trimethadione) 6–13 days (dimethadione) |
| Excretion | Renal |
| Identifiers | |
|
|
| CAS Number | 127-48-0 |
| PubChem (CID) | 5576 |
| IUPHAR/BPS | 7316 |
| DrugBank | DB00347 |
| ChemSpider | 5374 |
| UNII | R7GV3H6FQ4 |
| KEGG | D00392 |
| ChEMBL | CHEMBL695 |
| ECHA InfoCard | 100.004.406 |
| Chemical and physical data | |
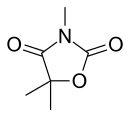
| Formula | C6H9NO3 |
| Molar mass | 143.141 g/mol |
| 3D model (Jmol) | Interactive image |
|
|
|
|
| (verify) | |
Trimethadione is an oxazolidinedione anticonvulsant. It is most commonly used to treat epileptic conditions that are resistant to other treatments.
Fetal trimethadione syndrome[edit]
If administered during pregnancy, fetal trimethadione syndrome may result causing facial dysmorphism (short upturned nose, slanted eyebrows), cardiac defects, intrauterine growth restriction (IUGR), and mental retardation. The fetal loss rate while using trimethadione has been reported to be as high as 87%.[1]
References[edit]
- ^ Teratology and Drug Use During Pregnancy Retrieved January 2007
External links[edit]
| This anticonvulsant-related article is a stub. You can help Wikipedia by expanding it. |