Clonazolam
From Wikipedia, the free encyclopedia
 |
|
 |
|
| Systematic (IUPAC) name | |
|---|---|
|
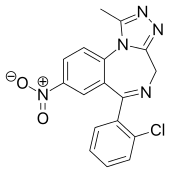
6-(2-chlorophenyl)-1-methyl-8-nitro-4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazepine
|
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | 33887-02-4 |
| PubChem | CID 12317881 |
| ChemSpider | 15468596 |
| Chemical data | |
| Formula | C17H12ClN5O2 |
| Molar mass | 353.07 g/mol |
| 3D model (Jmol) | Interactive image |
|
|
|
|
Clonazolam (also known as clonitrazolam) is a benzodiazepine that has been sold online as a designer drug.[1][2][3][4][5]
The synthesis of clonazolam was first reported in 1971 and the drug was described as the most active compound in the series tested.[6][7]
Clonazolam is reputed to be highly potent, and concerns have been raised that clonazolam and flubromazolam in particular may pose comparatively higher risks than other designer benzodiazepines, due to their ability to produce strong sedation and amnesia at oral doses of as little as 0.5 mg.[8]
Legality[edit]
Sweden's public health agency suggested classifying clonazolam as hazardous substance on June 1, 2015.[9]
See also[edit]
References[edit]
- ^ Laura M. Huppertz; Philippe Bisel; Folker Westphal; Florian Franz; Volker Auwärter; Bjoern Moosmann (July 2015). "Characterization of the four designer benzodiazepines clonazolam, deschloroetizolam, flubromazolam, and meclonazepam, and identification of their in vitro metabolites". Forensic Toxicology. 33 (2): 388–395. doi:10.1007/s11419-015-0277-6.
- ^ Markus R. Meyer; Madeleine Pettersson Bergstrand; Anders Helander; Olof Beck (May 2016). "Identification of main human urinary metabolites of the designer nitrobenzodiazepines clonazolam, meclonazepam, and nifoxipam by nano-liquid chromatography-high-resolution mass spectrometry for drug testing purposes". Analytical and Bioanalytical Chemistry. 408 (13): 3571–3591. doi:10.1007/s00216-016-9439-6. PMID 27071765.
- ^ M. Chaslot; S. El Balkhi; T. Robin; J. Morichon; N. Picard; F. Saint-Marcoux (June 2016). "Exploration des métabolites de 8 benzodiazépines de synthèse". Toxicologie Analytique et Clinique. 28 (2): S32. doi:10.1016/j.toxac.2016.03.053.
- ^ Madeleine Pettersson Bergstrand; Anders Helander; Therese Hansson; Olof Beck (2016). "Detectability of designer benzodiazepines in CEDIA, EMIT II Plus, HEIA, and KIMS II immunochemical screening assays". Drug Testing and Analysis. doi:10.1002/dta.2003. PMID 27366870.
- ^ Høiseth, Gudrun; Tuv, Silja Skogstad; Karinen, Ritva (2016). "Blood concentrations of new designer benzodiazepines in forensic cases". Forensic Science International. doi:10.1016/j.forsciint.2016.09.006.
- ^ Jackson B. Hester Jr.; Allan D. Rudzik; Bharat V. Kamdar (November 1971). "6-Phenyl-4H-s-triazolo[4,3-a] [1,4]benzodiazepines which have central nervous system depressant activity". Journal of Medicinal Chemistry. 14 (11): 1078–1081. doi:10.1021/jm00293a015. PMID 5165540.
- ^ René Borer; Max Gerecke; Emilio Kyburz (22 October 1986). "Patent EP 0072029 B1 - Triazolobenzazepines, process and intermediates for their preparation and medicines containing them". Retrieved 6 August 2015.
- ^ Bjoern Moosmann; Leslie A King; Volker Auwärter (June 2015). "Designer benzodiazepines: A new challenge". World Psychiatry. 14 (2): 248. doi:10.1002/wps.20236. PMC 4471986
 . PMID 26043347.
. PMID 26043347. - ^ "23 nya ämnen kan klassas som narkotika eller hälsofarlig vara" (in Swedish). Folkhälsomyndigheten. Retrieved 6 August 2015.
| This sedative-related article is a stub. You can help Wikipedia by expanding it. |