Alphenal
From Wikipedia, the free encyclopedia
(Redirected from Phenallymal)
 |
|
 |
|
| Clinical data | |
|---|---|
| Routes of administration |
Oral |
| ATC code | none |
| Legal status | |
| Legal status |
|
| Identifiers | |
|
|
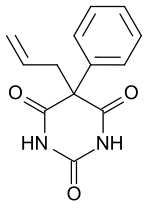
| Synonyms | 5-Phenyl-5-allylbarbituric acid |
| CAS Number | 115-43-5 |
| PubChem (CID) | 8274 |
| ChemSpider | 7975 |
| UNII | 7T7L08Q9JI |
| ECHA InfoCard | 100.003.718 |
| Chemical and physical data | |
| Formula | C13H12N2O3 |
| Molar mass | 244.246 g/mol |
| 3D model (Jmol) | Interactive image |
|
|
|
|
| (verify) | |
Alphenal (Alphenal, Efrodal, Prophenal, Sanudorm), also known as 5-allyl-5-phenylbarbituric acid, is a barbiturate derivative developed in the 1920s.[1] It has primarily anticonvulsant properties, and was used occasionally for the treatment of epilepsy or convulsions, although not as commonly as better known barbiturates such as phenobarbital.[2][3] [4][5]
LD50: Mouse (Oral): 280 mg/kg
References[edit]
- ^ DE Patent 526854
- ^ Carissimi M, Nuovi Barbiturici Alogenati. Farmaco. Ediozione scientifica. 17(6):390-413. (1962).
- ^ Martin, J. R.; Godel, T.; Hunkeler, W.; Jenck, F.; Moreau, J.-L.; Sleight, A. J.; Widmer, U. (2000). "Psychopharmacological Agents". doi:10.1002/0471238961.1619250313011820.a01.
- ^ Hans Brandenberger; Robert A. A. Maes (1997). Analytical Toxicology: For Clinical, Forensic, and Pharmaceutical Chemists. Walter de Gruyter. p. 348. ISBN 978-3-11-010731-9. Retrieved 19 May 2012.
- ^ Pelayo Camps García; Santiago Vázquez Cruz; Carmen Escolano Mirón (28 January 2005). Fundamentos de síntesis de fármacos. Edicions Universitat Barcelona. p. 161. ISBN 978-84-475-2876-9. Retrieved 19 May 2012.
| This anticonvulsant-related article is a stub. You can help Wikipedia by expanding it. |
| This sedative-related article is a stub. You can help Wikipedia by expanding it. |