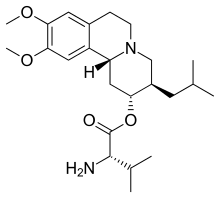
Valbenazine
 |
|
| Clinical data | |
|---|---|
| ATC code | none |
| Legal status | |
| Legal status |
|
| Identifiers | |
|
|
| Synonyms | NBI-98854 |
| CAS Number | 1025504-45-3 |
| PubChem (CID) | 24795069 |
| ChemSpider | 28536134 |
| UNII | 54K37P50KH |
| KEGG | D10675 |
| ChEMBL | CHEMBL2364639 |
| Chemical and physical data | |
| Formula | C24H38N2O4 |
| Molar mass | 418.58 g·mol−1 |
| 3D model (Jmol) | Interactive image |
|
|
|
|
Valbenazine (INN,[1]:114 proposed trade name Ingrezza) is an experimental drug being investigated for use in the treatment of tardive dyskinesia[2][3] and Tourette syndrome.[4][5] It acts as a vesicular monoamine transporter 2 (VMAT2) inhibitor.[6]
Contents
Pharmacology[edit]
Mechanism of action[edit]
Valbenazine is known to cause reversible reduction of dopamine release by selectively inhibiting pre-synaptic human vesicular monoamine transporter type 2 (VMAT2). In vitro, valbenazine shows great selectivity for VMAT2 and little to no affinity for VMAT1 or other monoamine receptors.[7] Although the exact cause of tardive dyskinsia is unknown, it is hypothesized that it may result from neuroleptic-induced dopamine hypersensitivity.[8] By selectively reducing the ability of VMAT2 to load dopamine into synaptic vesicles,[9] the drug reduces overall levels of available dopamine in the synaptic cleft, ideally alleviating the symptoms associated with dopamine hypersensitivity. The importance of valbenazine selectivity inhibiting VMAT2 over other monoamine transporters is that VMAT2 is mainly involved with the transport of dopamine, and to a much lesser extent other monoamines such as norepinephrine, serotonin, and histamine. This selectivity is likely to reduce the likelihood of "off-target" adverse effects which may result from the upstream inhibition of these other monoamines.[10]
Society and culture[edit]
Commercial aspects[edit]
Valbenazine is produced by Neurocrine Biosciences, a company based in San Diego. In addition to the late-stage clinical trials studying valbenazine, Neurocrine Biosciences (partnered with AbbVie Inc.) also has another product, elagolix (a hormone antagonist), undergoing clinical trials.[11] Following the initiation of these trials, on 5 May 2016 Neurocrine reported revenues of $15 million for the first quarter of 2016.[12] The company now focuses on filing the valbenazine new drug application as they prepare for the commercial launch of the drug for the treatment of tardive dyskinesia.Neurocrine's expenses have risen steadily since May 2015, primarily due to the pre-commercialization activities for valbenazine. [13]
Intellectual property[edit]
While Neurocrine Biosciences does not currently hold a final patent for valbenazine or elagolix, they do hold a patent for the VMAT2 inhibitor [9,10-dimethoxy-3-(2-methylpropyl)-1H,2H,3H,4H,6H,7H,11bH-pyrido-[2,1-a]isoquinolin-2-yl]methanol and related compounds, which includes valbenazine.[14]
See also[edit]
References[edit]
- ^ "International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary Names: List 71" (PDF). World Health Organization. Retrieved 18 November 2016.
- ^ Ben Adams (Aug 30, 2016). "Neurocrine submits valbenazine NDA early, set for 2017 approval". fiercebiotech.com.
- ^ "Safety and Tolerability Study of NBI-98854 for the Treatment of Tardive Dyskinesia - Full Text View - ClinicalTrials.gov". clinicaltrials.gov. Retrieved 2016-11-13.
- ^ "Tourette Syndrome Clinical Trials | Neurocrine Biosciences". www.neurocrine.com. Retrieved 2016-11-13.
- ^ "Safety and Efficacy Study of NBI-98854 in Adults With Tourette Syndrome - Full Text View - ClinicalTrials.gov". clinicaltrials.gov. Retrieved 2016-11-13.
- ^ O'Brien, C. F.; Jimenez, R; Hauser, R. A.; Factor, S. A.; Burke, J; Mandri, D; Castro-Gayol, J. C. (2015). "NBI-98854, a selective monoamine transport inhibitor for the treatment of tardive dyskinesia: A randomized, double-blind, placebo-controlled study". Movement Disorders. 30 (12): 1681–7. doi:10.1002/mds.26330. PMC 5049616
 . PMID 26346941.
. PMID 26346941. - ^ "NBI-98854 – VMAT2 Inhibitor | Tics in Children Treatment | Neurocrine Biosciences". www.neurocrine.com. Retrieved 2016-11-13.
- ^ "tardive-dyskinesia". www.priory.com. Retrieved 2016-11-13.
- ^ Purves, Dale, et al. Neuroscience. Sinauer Associates. 087893646
- ^ "NBIX: NDA for Valbenazine in Tardive Dyskinesia to be Filed in 2016…". Retrieved 2016-11-13.
- ^ "Endocrine & Movement Disorder R&D | About | Neurocrine Biosciences". www.neurocrine.com. Retrieved 2016-11-14.
- ^ "NBIX: NDA for Valbenazine in Tardive Dyskinesia to be Filed in 2016…". Retrieved 2016-11-20.
- ^ "Press Release | Neurocrine Biosciences, Inc.". phoenix.corporate-ir.net. Retrieved 2016-11-20.
- ^ "[9,10-dimethoxy-3-(2-methylpropyl)-1h,2h,3h,4h,6h,7h,11bh-pyrido-[2,1-a]isoquinolin-2-yl]methanol And Compounds, Compositions And Methods Relating Thereto". Retrieved 2016-11-20.
| This drug article relating to the nervous system is a stub. You can help Wikipedia by expanding it. |