Zeranol
 |
|
| Clinical data | |
|---|---|
| Routes of administration |
Oral |
| Identifiers | |
|
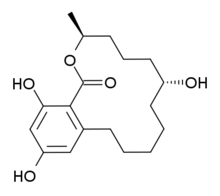
Systematic (IUPAC) name: (3S,7R)-7,14,16-Trihydroxy-3-methyl-3,4,5,6,7,8,9,10,11,12-decahydro-1H-2-benzoxacyclotetradecin-1-one
|
|
| Synonyms | α-Zearalanol; Zearalanol; MK-188; P-1496 |
| CAS Number | 26538-44-3 |
| PubChem (CID) | 2999413 |
| ChemSpider | 2271133 |
| UNII | 76LO2L2V39 |
| ChEMBL | CHEMBL371463 |
| Chemical and physical data | |
| Formula | C18H26O5 |
| Molar mass | 322.40 g·mol−1 |
| 3D model (Jmol) | Interactive image |
|
|
|
|
| |
|
Zeranol (INN, USAN, BAN) (brand names Frideron, Ralabol, Ralgro, Ralone, Zerano; developmental code names MK-188, P-1496), or zearanol, also known as α-zearalanol or simply zearalanol, is a naturally occurring mycoestrogen found in fungi in the Fusarium genus and a non-steroidal estrogen used mainly as an anabolic agent in veterinary medicine.[1][2][3]
Zeranol is approved for use as a growth promoter in livestock, including beef cattle, under the brand name Ralgro (by Merck Animal Health) in the United States.[4] In Canada, it is approved for use in beef cattle only.[5] Its application is not approved for use in the European Union. However, it is marketed under the brand name Ralone in Spain.[2]
Although zeranol may increase cancer cell proliferation in already existing breast cancer,[6] dietary exposure from the use of zeranol-containing implants in cattle is insignificant.[7] Zeranol may be found as a contaminant in fungus-infected crops. It is 3 to 4 times more potent as an estrogen than the related compound zearalenone.[8]
See also[edit]
References[edit]
- ^ J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 350–. ISBN 978-1-4757-2085-3.
- ^ a b Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 1105–. ISBN 978-3-88763-075-1.
- ^ I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 295–. ISBN 978-94-011-4439-1.
- ^ Nichols, Wade; Hutcheson, John; Streeter, Marshall; Corrigan, Mark; Nuttelman, Brandon. "Implant Strategies for Finishing Cattle using Revalor® (trenbolone acetate and estradiol), Finaplix® (trenbolone) and/or Ralgro® (zeranol)" (PDF). Merck Animal Health.
- ^ Health Canada, Questions and Answers - Hormonal Growth Promoters
- ^ Xu, Pingping; Ye, Weiping; Jen, Robert; Lin, Shu-Hong; Kuo, Chieh-Ti; Lin, Young C. (2009-11-01). "Mitogenic activity of zeranol in human breast cancer cells is enhanced by leptin and suppressed by gossypol". Anticancer Research. 29 (11): 4621–4628. ISSN 1791-7530. PMID 20032412.
- ^ Lindsay DG (August 1985). "Zeranol--a 'nature-identical' oestrogen?". Food Chem Toxicol. 23 (8): 767–74. PMID 2931335.
- ^ Mirocha, CJ; Schauerhamer, B; Christensen, CM; Niku-Paavola, ML; Nummi, M (1979). "Incidence of zearalenol (Fusarium mycotoxin) in animal feed". Applied and Environmental Microbiology. 38 (4): 749–50. PMC 243572
 . PMID 161492.
. PMID 161492.