Apigenin
 |
|
 |
|
| Names | |
|---|---|
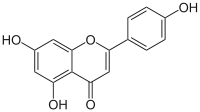
| IUPAC name
5,7-Dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one
|
|
| Other names
Apigenine; Chamomile; Apigenol; Spigenin; Versulin; 4′,5,7-Trihydroxyflavone; C.I. Natural Yellow 1
|
|
| Identifiers | |
| 520-36-5 |
|
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:18388 |
| ChEMBL | ChEMBL28 |
| ChemSpider | 4444100 |
| DrugBank | DB07352 |
| ECHA InfoCard | 100.007.540 |
| 4136 | |
| KEGG | C01477 |
| PubChem | 5280443 |
|
|
|
|
| Properties | |
| C15H10O5 | |
| Molar mass | 270.24 g·mol−1 |
| Appearance | Yellow crystalline solid |
| Melting point | 345 to 350 °C (653 to 662 °F; 618 to 623 K) |
| UV-vis (λmax) | 267, 296sh, 336 nm in methanol[2] |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |
Apigenin (4′,5,7-trihydroxyflavone), found in many plants, is a natural product belonging to the flavone class that is the aglycone of several naturally occurring glycosides. It is a yellow crystalline solid that has been used to dye wool.
Contents
Pharmacology[edit]
In in vitro experiments and animal studies, a variety of potential biological activities of apigenin have been identified, but its effects on human health are unknown.
Apigenin induces autophagy (a kind of cellular waste-recycling system) in leukemia cells, which may support a possible chemopreventive role. Induced autophagy interfers with the action of the chemotherapy drug vincristine.[3] Apigenin is a potent inhibitor of CYP2C9,[4] an enzyme responsible for the metabolism of many pharmaceutical drugs in the body. Apigenin dimers can reverse the highest level of drug resistance found in cancer stem cells.[5]
Apigenin has been shown to prevent renal damage caused by ciclosporin in rats, associated with reduced expression of the cell death mediator bcl-2 in histopathological sections.[6] Ciclosporin enhances the expression of transforming growth factor-β in the rat kidney, which signifies accelerated apoptosis. Therefore, transforming growth factor-β and apoptotic index may be used to assess apigenin and its effect on ciclosporin-induced renal damage.[7]
Apigenin acts as a monoamine transporter activator, one of the few chemicals demonstrated to possess this property.[8] Apigenin is a ligand for central benzodiazepine receptors that competitively inhibited the binding of flunitrazepam with a Ki of 4 μM, exerting anxiolytic and slight sedative effects.[9] Apigenin shows second-order positive modulatory activity at GABAA receptors.[10][11] It has also effects on adenosine receptors[12] and blocks NMDA receptors (IC50 = 10 μM).[11] In addition, like various other flavonoids, apigenin has been found to possess nanomolar affinity for the opioid receptors (Ke = 410 nM, 970 nM, and 410 nM for the μ-, δ-, and κ-opioid receptors, respectively), acting as a non-selective antagonist of all three opioid receptors.[13]
In vitro studies have shown that apigenin may be toxic to red blood cells.[14]
Potential health benefits[edit]
Apigenin may also stimulate adult neurogenesis, with at least one study claiming that apigenin "stimulate[s] adult neurogenesis in vivo and in vitro, by promoting neuronal differentiation" and may be useful "for stimulating adult neurogenesis and for the treatment of neurological diseases, disorders and injuries, by stimulating the generation of neuronal cells in the adult brain." While potentially promising, the study used rats and its effects have yet to be demonstrated in humans.[15]
Apigenin readily crosses the blood-brain barrier and has not demonstrated toxicity at high doses.[16] It could thus prevent amyloid beta deposition and tau phosphorylation due to neuroinflammation, which can lead to Alzheimer's disease.[16]
Through effects on cell signaling, inflammation, cell cycle, and protease production, apigenin has demonstrated effectiveness against a wide range of cancer types, while not showing toxicity to normal cells.[17][18]
Sources in nature[edit]
Apigenin is found in many fruits and vegetables, but parsley, celery, celeriac, and chamomile tea are the most common sources.[19] Apigenin is particularly abundant in the flowers of chamomile plants, constituting 68% of total flavonoids.[16]
Glycosides[edit]
The naturally occurring glycosides formed by the combination of apigenin with sugars include:
- Apiin (apigenin 7-O-apioglucoside), isolated from parsley[20] and celery
- Apigetrin (apigenin 7-glucoside), found in dandelion coffee
- Vitexin (apigenin 8-C-glucoside)
- Isovitexin (apigenin 6-C-glucosid)
- Rhoifolin (apigenin 7-O-neohesperidoside)
- Schaftoside (apigenin 6-C-glucoside 8-C-arabinoside)
See also[edit]
References[edit]
- ^ Merck Index, 11th Edition, 763.
- ^ The Systematic Identification of Flavonoids. Mabry et al, 1970, page 81
- ^ RR Ruela-de-Sousa; GM Fuhler; N Blom; CV Ferreira; H Aoyama; MP Peppelenbosch (2010). "Cytotoxicity of apigenin on leukemia cell lines: implications for prevention and therapy". Cell Death and Disease. 1 (e19): 1–11. doi:10.1038/cddis.2009.18. PMC 3032507
 . PMID 21364620.
. PMID 21364620. - ^ Si Dayong; Wang Y; Zhou Y-H; Guo Y; Wang J; Zhou H; Li Z-S; Fawcett JP (March 2009). "Mechanism of CYP2C9 inhibition by flavones and flavonols" (PDF). Drug Metabolism and Disposition. 37 (3): 629–634. doi:10.1124/dmd.108.023416. PMID 19074529.
- ^ Apigenin, a natural metabolite found in plants and vegetables, is poised to improve chemotherapy significantly
- ^ Srikumar Chakravarthi; Chong Fu Wen; HS Nagaraja (2009). "Apoptosis and expression of bcl-2 in cyclosporin induced renal damage and its reversal by beneficial effects of 4,5,7 - Trihydroxyflavone" (PDF). Journal of Analytical Bio Science. 32 (4): 320–327.
- ^ Chong FW; Srikumar Chakravarthi; HS Nagaraja; PM Thanikachalam; Nagarajah Lee (2009). "Expression of Transforming Growth factor-β and determination of Apoptotic Index in histopathological sections for assessment of the effects of Apigenin (4',5',7'- trihydroxyflavone) on Cyclosporine A induced renal damage". Malaysian Journal of Pathology. 31 (1): 35–43. PMID 19694312.
- ^ Zhao, G; Qin, GW; Wang, J; Chu, WJ; Guo, LH (2010). "Functional activation of monoamine transporters by luteolin and apigenin isolated from the fruit of Perilla frutescens (L.) Britt". Neurochemistry international. 56 (1): 168–76. doi:10.1016/j.neuint.2009.09.015. PMID 19815045.
- ^ Viola, H; Wasowski, C; Levi De Stein, M; Wolfman, C; Silveira, R; Dajas, F; Medina, JH; Paladini, AC (1995). "Apigenin, a component of Matricaria recutita flowers, is a central benzodiazepine receptors-ligand with anxiolytic effects". Planta Medica. 61 (3): 213–6. doi:10.1055/s-2006-958058. PMID 7617761.
- ^ Campbell, Erica L.; Chebib, Mary; Johnston, Graham A. R. (2004-10-15). "The dietary flavonoids apigenin and (-)-epigallocatechin gallate enhance the positive modulation by diazepam of the activation by GABA of recombinant GABA(A) receptors". Biochemical Pharmacology. 68 (8): 1631–1638. doi:10.1016/j.bcp.2004.07.022. ISSN 0006-2952. PMID 15451406.
- ^ a b Losi, Gabriele; Puia, Giulia; Garzon, Giorgio; de Vuono, Maria C.; Baraldi, Mario (2004-10-11). "Apigenin modulates GABAergic and glutamatergic transmission in cultured cortical neurons". European Journal of Pharmacology. 502 (1-2): 41–46. doi:10.1016/j.ejphar.2004.08.043. ISSN 0014-2999. PMID 15464088.
- ^ Jacobson, Kenneth A.; Moro, Stefano; Manthey, John A.; West, Patrick L.; Ji, Xiao-Duo (2002-01-01). "Interactions of flavones and other phytochemicals with adenosine receptors". Advances in Experimental Medicine and Biology. 505: 163–171. ISSN 0065-2598. PMC 3429336
 . PMID 12083460.
. PMID 12083460. - ^ Katavic PL, Lamb K, Navarro H, Prisinzano TE (August 2007). "Flavonoids as opioid receptor ligands: identification and preliminary structure-activity relationships". J. Nat. Prod. 70 (8): 1278–82. doi:10.1021/np070194x. PMC 2265593
 . PMID 17685652.
. PMID 17685652. - ^ Zbidah, M; Lupescu, A; Jilani, K; Fajol, A; Michael, D; Qadri, SM; Lang, F (2012). "Apigenin-induced suicidal erythrocyte death". Journal of Agricultural and Food Chemistry. 60 (1): 533–8. doi:10.1021/jf204107f. PMID 22132906.
- ^ Taupin, P (2009). "Apigenin and related compounds stimulate adult neurogenesis. Mars, Inc., the Salk Institute for Biological Studies: WO2008147483". Expert opinion on therapeutic patents. 19 (4): 523–7. doi:10.1517/13543770902721279. PMID 19441930.
- ^ a b c Venigalla M, Gyengesi E, Münch G (2015). "Curcumin and Apigenin - novel and promising therapeutics against chronic neuroinflammation in Alzheimer's disease". Neural Regeneration Research. 10 (8): 1181–1185. doi:10.4103/1673-5374.162686. PMC 4590215
 . PMID 26487830.
. PMID 26487830. - ^ Shukla S1, Gupta S (2010). "Apigenin: a promising molecule for cancer prevention". Journal of Agricultural and Food Chemistry. 27 (6): 962–978. doi:10.1007/s11095-010-0089-7. PMC 2874462
 . PMID 20306120.
. PMID 20306120. - ^ Srivastava JK1, Gupta S (2007). "Antiproliferative and apoptotic effects of chamomile extract in various human cancer cells". Pharmaceutical Research (journal). 55 (23): 9470–9478. doi:10.1021/jf071953k. PMID 17939735.
- ^ The compound in the Mediterranean diet that makes cancer cells 'mortal' Emily Caldwell, Medical Express, May 20, 2013.
- ^ Meyer, Hellen; Bolarinwa, Adrian; Wolfram, Guenther; Linseisen, Jakob (2006). "Bioavailability of Apigenin from Apiin-Rich Parsley in Humans". Annals of Nutrition and Metabolism. 50 (3): 167–72. doi:10.1159/000090736. PMID 16407641.