SER-601
From Wikipedia, the free encyclopedia
 |
|
| Identifiers | |
|---|---|
|
|
| CAS Number | 1048038-90-9 |
| PubChem (CID) | 25034551 |
| ChemSpider | 24606023 |
| ChEMBL | CHEMBL502276 |
| Chemical and physical data | |
| Formula | C28H38N2O2 |
| Molar mass | 434.612 g/mol |
| 3D model (Jmol) | Interactive image |
|
|
|
|
| |
|
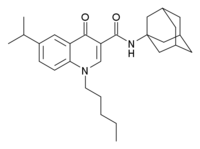
SER-601 (COR-167) is a drug which acts as a potent and selective cannabinoid CB2 receptor agonist, based on a quinolone-3-carboxylic acid core structure, with 190x selectivity for CB2 over the related CB1 receptor. It has analgesic effects in animal studies, as well as neuroprotective effects,[1] but without cannabis-like behavioural effects due to its low affinity for CB1.[2] A number of related compounds are known, almost all of which have high selectivity for CB2.[3]
See also[edit]
References[edit]
- ^ Contartese, A.; Valoti, M.; Corelli, F.; Pasquini, S.; Mugnaini, C.; Pessina, F.; Aldinucci, C.; Sgaragli, G.; Frosini, M. (2012). "A novel CB2 agonist, COR167, potently protects rat brain cortical slices against OGD and reperfusion injury". Pharmacological Research. 66 (6): 555–563. doi:10.1016/j.phrs.2012.08.003. PMID 23036353.
- ^ Pasquini S, et al. (August 2008). "Investigations on the 4-quinolone-3-carboxylic acid motif. 2. Synthesis and structure-activity relationship of potent and selective cannabinoid-2 receptor agonists endowed with analgesic activity in vivo". Journal of Medicinal Chemistry. 51 (16): 5075–84. doi:10.1021/jm800552f. PMID 18680276.
- ^ Pasquini S, et al. (August 2010). "Investigations on the 4-quinolone-3-carboxylic acid motif. 3. Synthesis, structure-affinity relationships, and pharmacological characterization of 6-substituted 4-quinolone-3-carboxamides as highly selective cannabinoid-2 receptor ligands". Journal of Medicinal Chemistry. 53 (16): 5915–28. doi:10.1021/jm100123x. PMID 20718492.
| This cannabinoid related article is a stub. You can help Wikipedia by expanding it. |