Arachidonyl-2'-chloroethylamide
From Wikipedia, the free encyclopedia
 |
|
| Names | |
|---|---|
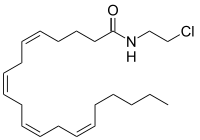
| IUPAC name
N-(2-Chloroethyl)-5Z,8Z,11Z,14Z-eicosatetraenamide
|
|
| Other names
Arachidonyl-2'-chloroethylamide; ACEA
|
|
| Identifiers | |
| 220556-69-4 |
|
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL151167 |
| ChemSpider | 4470547 |
| 738 | |
| PubChem | 5311006 |
|
|
|
|
| Properties | |
| C22H36ClNO | |
| Molar mass | 365.99 g·mol−1 |
| Solubility in other solvents | soluble in ethanol, chloroform, THF and DMSO |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |
Arachidonyl-2'-chloroethylamide (ACEA) is a synthetic agonist of the cannabinoid receptor 1 (CB1R). ACEA is considered to be a selective cannabinoid agonist as it binds primarily to the CB1R and has low affinity to the cannabinoid receptor 2 (CB2R) (Ki = 1.4 nM for CB1R; Ki = 3100 nM for CB2R). [1]
References[edit]
- ^ Hillard, CJ; Manna, S; Greenberg, MJ; Dicamelli, R; Ross, RA; Stevenson, LA; Murphy, V; Pertwee, RG; Campbell, WB (1999). "Synthesis and characterization of potent and selective agonists of the neuronal cannabinoid receptor (CB1)". The Journal of Pharmacology and Experimental Therapeutics. 289 (3): 1427–33. PMID 10336536.