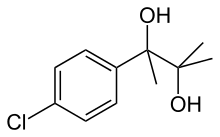
Phenaglycodol
 |
|
| Clinical data | |
|---|---|
| ATC code | None |
| Identifiers | |
|
|
| CAS Number | 79-93-6 |
| PubChem (CID) | 6617 |
| ChemSpider | 6365 |
| Chemical and physical data | |
| Formula | C11H15ClO2 |
| Molar mass | 214.689 g/mol |
| 3D model (Jmol) | Interactive image |
|
|
|
|
Phenaglycodol (INN, BAN; brand names Acalmid, Acalo, Alterton, Atadiol, Felixyn, Neotran, Pausital, Remin, Sedapsin, Sinforil, Stesil, Ultran)[1] is a drug described as a tranquilizer or sedative which has anxiolytic and anticonvulsant properties.[2][3] It is related structurally and pharmacologically to meprobamate, though it is not a carbamate.[4][5]
Contents
Synthesis[edit]

p-Chloroacetophenone and NaCN are reacted together to give the corresponding cyanohydrin (cf Strecker synthesis). The cyano group is then hydrated in acid to the corresponding amide, thus p-chloroatrolactamide (4) is formed. The amide group is then further hydrolyzed with a 2nd equivalent of water in concentrated lye to p-chloroatrolactic acid (5); this is then esterified to Ethyl p-chloroatrolactate (6). Finally, nucleophilic addition a couple of equivalents of MeMgI are added to the ester give Phenaglycodol (7) crystals.
Notes[edit]
- See "Novel trifluoromethyl derivatives of substituted diols" U.S. Patent 3,134,819 also.
See also[edit]
References[edit]
- ^ Earl Usdin; Daniel H. Efron; National Institute of Mental Health (U.S.) (1972). Psychotropic drugs and related compounds. National Institute of Mental Health; [for sale by the Supt. of Docs., U.S. Govt. Print. Off., Washington.
- ^ Julius Vida (19 July 2013). Anticonvulsants. Elsevier. pp. 578–. ISBN 978-0-323-14395-0.
- ^ Lester M. Haddad; James F. Winchester (1983). Clinical Management of Poisoning and Drug Overdose. Saunders. ISBN 978-0-7216-4447-9.
- ^ Victor Alexander Drill (1958). Pharmacology in Medicine: A Collaborative Textbook. McGraw-Hill.
- ^ Harry Beckman (1961). Pharmacology; the nature, action and use of drugs. Saunders.
| This drug article relating to the nervous system is a stub. You can help Wikipedia by expanding it. |