DS-1 (drug)
From Wikipedia, the free encyclopedia
 |
|
| Systematic (IUPAC) name | |
|---|---|
|
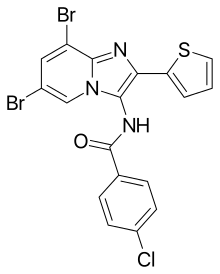
4-chloro-N-(6,8-dibromo-2-thiophen-2-ylimidazo[1,2-a]pyridin-3-yl)benzamide
|
|
| Identifiers | |
| CAS Number | 372497-52-4 |
| PubChem | CID 979735 |
| IUPHAR/BPS | 4183 |
| ChemSpider | 849261 |
| Chemical data | |
| Formula | C18H10Br2ClN3OS |
| Molar mass | 511.617 g/mol |
| 3D model (Jmol) | Interactive image |
|
|
| (verify) | |
DS-1 is a drug from the imidazopyridine family, which is the first drug developed that acts as a GABAA receptor positive allosteric modulator (PAM) selective for the α4β3δ subtype, which is not targeted by other GABAA receptor PAMs such as the benzodiazepines or other nonbenzodiazepine drugs. Novel selective drugs such as DS-1 should prove useful in the study of this receptor subtype.[1]
See also[edit]
References[edit]
- ^ Wafford, KA; Van Niel, MB; Ma, QP; Horridge, E; Herd, MB; Peden, DR; Belelli, D; Lambert, JJ (2009). "Novel compounds selectively enhance delta subunit containing GABA a receptors and increase tonic currents in thalamus". Neuropharmacology. 56 (1): 182–9. doi:10.1016/j.neuropharm.2008.08.004. PMID 18762200.
| This sedative-related article is a stub. You can help Wikipedia by expanding it. |