Heptabarb
From Wikipedia, the free encyclopedia
 |
|
| Systematic (IUPAC) name | |
|---|---|
|
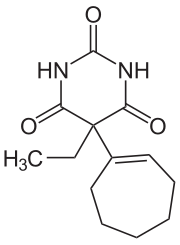
5-cyclohept-1-en-1-yl-5-ethylpyrimidine-2,4,6(1H,3H,5H)-trione
|
|
| Clinical data | |
| Pregnancy category |
|
| Routes of administration |
Oral[1] |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 83%[1] |
| Metabolism | Hepatic |
| Biological half-life | 6.1-11.2 hours[1] |
| Excretion | Renal[1] |
| Identifiers | |
| CAS Number | 509-86-4 |
| ATC code | N05CA11 (WHO) |
| PubChem | CID 10518 |
| DrugBank | DB01354 |
| ChemSpider | 10081 |
| UNII | V10R70ML23 |
| KEGG | C17725 |
| ChEMBL | CHEMBL468837 |
| Synonyms | G-475 |
| Chemical data | |
| Formula | C13H18N2O3 |
| Molar mass | 250.294 g/mol |
| 3D model (Jmol) | Interactive image |
|
|
|
|
| (verify) | |
Heptabarb (INN; Eudan, Medapan, Medomin, Noctyn), also known as heptabarbitone (BAN) or heptabarbital, is a sedative and hypnotic drug of the barbiturate family.[2][3] It was used in Europe for the treatment of insomnia from the 1950s onwards, but has since been discontinued.[2][3]
See also[edit]
References[edit]
- ^ a b c d Breimer DD, de Boer AG (December 1975). "Pharmacokinetics and relative bioavailability of heptabarbital and heptabarbital sodium after oral administration to man". European Journal of Clinical Pharmacology. 9 (2-3): 169–78. doi:10.1007/bf00614014. PMID 9299.
- ^ a b C. R Ganellin; D. J Triggle; F.. Macdonald (1997). Dictionary of pharmacological agents. CRC Press. p. 1003. ISBN 978-0-412-46630-4. Retrieved 26 November 2011.
- ^ a b Index nominum 2000: international drug directory. Taylor & Francis US. 2000. p. 513. ISBN 978-3-88763-075-1. Retrieved 26 November 2011.
| This sedative-related article is a stub. You can help Wikipedia by expanding it. |