Avizafone
From Wikipedia, the free encyclopedia
 |
|
| Systematic (IUPAC) name | |
|---|---|
|
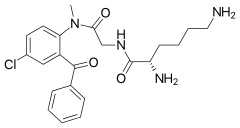
(2S)-2,6-diamino-N-{[(2-benzoyl-4-chlorophenyl)methylcarbamoyl]methyl}hexanamide
|
|
| Clinical data | |
| Routes of administration |
Intramuscular injection |
| Identifiers | |
| CAS Number | 65617-86-9 |
| ATC code | none |
| PubChem | CID 71968 |
| ChemSpider | 64974 |
| UNII | 65NK71K78P |
| ChEMBL | CHEMBL2103985 |
| Chemical data | |
| Formula | C22H27ClN4O3 |
| Molar mass | 430.928 g/mol |
| 3D model (Jmol) | Interactive image |
|
|
|
|
| |
|
Avizafone[1] (Pro-Diazepam) is a water-soluble prodrug of diazepam. It can be administered intramuscularly.
Avizafone is metabolised by enzymes in the blood to form the active drug diazepam. It is used mainly as an antidote to poisoning with organophosphate nerve agents.[2][3][4]
See also[edit]
References[edit]
- ^ GB Patent 1517164
- ^ Karlsson B, Lindgren B, Millquist E, Sandberg M, Sellstrom A. On the use of diazepam and pro-diazepam (2-benzoyl-4-chloro-N-methyl-N-lysylglycin anilide), as adjunct antidotes in the treatment of organophosphorus intoxication in the guinea-pig. Journal of Pharmacy and Pharmacology. 1990 Apr;42(4):247-51.
- ^ Lallement G, Renault F, Baubichon D, Peoc'h M, Burckhart MF, Galonnier M, Clarencon D, Jourdil N. Compared efficacy of diazepam or avizafone to prevent soman-induced electroencephalographic disturbances and neuropathology in primates: relationship to plasmatic benzodiazepine pharmacokinetics. Archives of Toxicology. 2000 Oct;74(8):480-6.
- ^ Taysse L, Calvet JH, Buee J, Christin D, Delamanche S, Breton P. Comparative efficacy of diazepam and avizafone against sarin-induced neuropathology and respiratory failure in guinea pigs: influence of atropine dose. Toxicology. 2003 Jun 30;188(2-3):197-209.
| This drug article relating to the nervous system is a stub. You can help Wikipedia by expanding it. |