- published: 01 Sep 2009
- views: 425816

Heat fusion (sometimes called heat welding, butt welding or simply fusion) is a welding process used to join two different pieces of a thermoplastic. This process involves heating both pieces simultaneously and pressing them together. The two pieces then cool together and form a permanent bond. When done properly, the two pieces become indistinguishable from each other. Dissimilar plastics can result in improper bonding.
This process is commonly used in plastic pressure pipe systems to join a pipe and fitting together, or to join a length of pipe directly to another length of pipe. Generally, polyolefins (such as polypropylene, polyethylene, and polybutylene) are used for these applications.
Butt welding is usually performed using one of several methods. The first, and most common, is butt welding or butt fusion, which is a type of hot plate welding. This technique involves heating two planed surfaces of thermoplastic material (typically polyethylene) against a heated surface. After a specified amount of time, the heating plate is removed and the two pieces are pressed together and allowed to cool under pressure, forming the desired bond. Butt welding outside of manufacturing is usually performed to join pipes.

Khan Academy is a non-profit educational organization created in 2006 by educator Salman Khan with the aim of providing a free, world-class education for anyone, anywhere. The organization produces short lectures in the form of YouTube videos. In addition to micro lectures, the organization's website features practice exercises and tools for educators. All resources are available for free to anyone around the world. The main language of the website is English, but the content is also available in other languages.
The founder of the organization, Salman Khan, was born in New Orleans, Louisiana, United States to immigrant parents from Bangladesh and India. After earning three degrees from the Massachusetts Institute of Technology (a BS in mathematics, a BS in electrical engineering and computer science, and an MEng in electrical engineering and computer science), he pursued an MBA from Harvard Business School.
In late 2004, Khan began tutoring his cousin Nadia who needed help with math using Yahoo!'s Doodle notepad.When other relatives and friends sought similar help, he decided that it would be more practical to distribute the tutorials on YouTube. The videos' popularity and the testimonials of appreciative students prompted Khan to quit his job in finance as a hedge fund analyst at Connective Capital Management in 2009, and focus on the tutorials (then released under the moniker "Khan Academy") full-time.

The enthalpy of fusion also known as (latent) heat of fusion is the change in enthalpy resulting from heating a given quantity of a substance to change its state from a solid to a liquid. The temperature at which this occurs is the melting point.
The 'enthalpy' of fusion is a latent heat, because during melting the introduction of heat cannot be observed as a temperature change, as the temperature remains constant during the process. The latent heat of fusion is the enthalpy change of any amount of substance when it melts. When the heat of fusion is referenced to a unit of mass, it is usually called the specific heat of fusion, while the molar heat of fusion refers to the enthalpy change per amount of substance in moles.
The liquid phase has a higher internal energy than the solid phase. This means energy must be supplied to a solid in order to melt it and energy is released from a liquid when it freezes, because the molecules in the liquid experience weaker intermolecular forces and so have a higher potential energy (a kind of bond-dissociation energy for intermolecular forces).

Heat capacity or thermal capacity is a measurable physical quantity equal to the ratio of the heat added to (or removed from) an object to the resulting temperature change. The SI unit of heat capacity is joule per kelvin 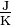 and the dimensional form is L2MT−2Θ−1. Specific heat is the amount of heat needed to raise the temperature of a certain mass by 1 degree Celsius.
and the dimensional form is L2MT−2Θ−1. Specific heat is the amount of heat needed to raise the temperature of a certain mass by 1 degree Celsius.
Heat capacity is an extensive property of matter, meaning it is proportional to the size of the system. When expressing the same phenomenon as an intensive property, the heat capacity is divided by the amount of substance, mass, or volume, so that the quantity is independent of the size or extent of the sample. The molar heat capacity is the heat capacity per unit amount (SI unit: mole) of a pure substance and the specific heat capacity, often simply called specific heat, is the heat capacity per unit mass of a material. Occasionally, in engineering contexts, the volumetric heat capacity is used.
Temperature reflects the average randomized kinetic energy of constituent particles of matter (e.g. atoms or molecules) relative to the centre of mass of the system, while heat is the transfer of energy across a system boundary into the body other than by work or matter transfer. Translation, rotation, and vibration of atoms represent the degrees of freedom of motion which classically contribute to the heat capacity of gases, while only vibrations are needed to describe the heat capacities of most solids , as shown by the Dulong–Petit law. Other, more exotic contributions can come from magnetic and electronic degrees of freedom in solids, but these rarely make substantial contributions.


Specific heat, heat of fusion and vaporization example | Chemistry | Khan Academy

Latent Heat of Fusion and Vaporization | Doc Physics
Heat of Fusion and Heat of Vaporization Explained

heat of fusion and vaporization problems

Heat of Fusion Calculation

Specific heat and latent leat of fusion and vaporization | Chemistry | Khan Academy

HDPE pipe welding using Heat Fusion

STYLE OF FUSION (坂見誠二&HIRO;) HEAT UP Vol.36 DANCE SHOWCASE

Latent heat of fusion and vaporisation

Calculating the specific latent heat of fusion of ice

Specific heat and phase changes: Calculating how much heat is needed to convert 200 g of ice at -10 degrees C to 110 degree steam. Watch the next lesson: https://www.khanacademy.org/science/chemistry/states-of-matter-and-intermolecular-forces/states-of-matter/v/chilling-water-problem?utm_source=YT&utm;_medium=Desc&utm;_campaign=chemistry Missed the previous lesson? https://www.khanacademy.org/science/chemistry/states-of-matter-and-intermolecular-forces/states-of-matter/v/specific-heat-and-latent-leat-of-fusion-and-vaporization-2?utm_source=YT&utm;_medium=Desc&utm;_campaign=chemistry Chemistry on Khan Academy: Did you know that everything is made out of chemicals? Chemistry is the study of matter: its composition, properties, and reactivity. This material roughly covers a first-year high sch...

We investigate why Home Depot is better than Lowe's. Or is it the other way around?

In this video I will explain the concept of latent heats of fusion and vaporization and work out some problems involving heat of fusion and heat of vaporization of water.


link-http://www.kentchemistry.com/links/Energy/HeatFusion.htm This short video takes demonstrates how to use the heat of fusion equation when solving heat problems. This is used for determining the amount of energy when an amount of ice is melted at 0 degrees Celsius.

Defining specific heat, heat of fusion, and heat of vaporization. How to calculate the amount of heat to change the temperature of water and the energy required to change for a phase change. Watch the next lesson: https://www.khanacademy.org/science/chemistry/states-of-matter-and-intermolecular-forces/states-of-matter/v/specific-heat-heat-of-fusion-and-vaporization?utm_source=YT&utm;_medium=Desc&utm;_campaign=chemistry Missed the previous lesson? https://www.khanacademy.org/science/chemistry/states-of-matter-and-intermolecular-forces/states-of-matter/v/states-of-matter-follow-up?utm_source=YT&utm;_medium=Desc&utm;_campaign=chemistry Chemistry on Khan Academy: Did you know that everything is made out of chemicals? Chemistry is the study of matter: its composition, properties, and reactivity...

One of the methods of joining two HDPE pipe lengths together is through heat fusion. The above video shows the process of HDPE welding though Heat fusion method in intricate detail.

使用Cam1 https://goo.gl/Y4EiUV Cam2 http://goo.gl/AA0h6V Cam3 http://goo.gl/ekGJo7 Lens https://goo.gl/LdOSf8 Gimbal https://goo.gl/W2GX3H 2 http://goo.gl/LE2zy6 (LUMIX DC-GH5 HC-VX980M FDR-X3000 M.ZUIKO DIGITAL ED 7-14mm F2.8 PRO Zhiyun Crane & Z1 Smooth C &more;)個人趣味の撮影ですが、年100万円前後使って撮影してるので、今年より撮影依頼される場合は1案件あたりの実費相当5,000円~の経費負担願います。SNS・メールに連絡下さい。撮影活動の維持存続にご理解ご協力願います。 連絡先:TW https://twitter.com/nekomon_KENN_39 FB https://www.facebook.com/nekomon1/ nekomon channelオフィシャル猫グッズ:https://www.ttrinity.jp/shop/nekomon/ I spend much money for shooting,but almost shooting without sponsor,please help my channel activity. movie Q&A; , DANCE EVENT report , etc:http://fanblogs.jp/dancemovie/category_5/ 撮影経費と負担額目安:http://fanblogs.jp/dancemovie/archive/145/0 ダンス動画チャンネル メイン DANCE MOVIE channel (Main contents ...


Physics

Specific heat and phase changes: Calculating how much heat is needed to convert 200 g of ice at -10 degrees C to 110 degree steam. Watch the next lesson: https://www.khanacademy.org/science/chemistry/states-of-matter-and-intermolecular-forces/states-of-matter/v/chilling-water-problem?utm_source=YT&utm;_medium=Desc&utm;_campaign=chemistry Missed the previous lesson? https://www.khanacademy.org/science/chemistry/states-of-matter-and-intermolecular-forces/states-of-matter/v/specific-heat-and-latent-leat-of-fusion-and-vaporization-2?utm_source=YT&utm;_medium=Desc&utm;_campaign=chemistry Chemistry on Khan Academy: Did you know that everything is made out of chemicals? Chemistry is the study of matter: its composition, properties, and reactivity. This material roughly covers a first-year high sch...

We investigate why Home Depot is better than Lowe's. Or is it the other way around?

In this video I will explain the concept of latent heats of fusion and vaporization and work out some problems involving heat of fusion and heat of vaporization of water.


link-http://www.kentchemistry.com/links/Energy/HeatFusion.htm This short video takes demonstrates how to use the heat of fusion equation when solving heat problems. This is used for determining the amount of energy when an amount of ice is melted at 0 degrees Celsius.

Defining specific heat, heat of fusion, and heat of vaporization. How to calculate the amount of heat to change the temperature of water and the energy required to change for a phase change. Watch the next lesson: https://www.khanacademy.org/science/chemistry/states-of-matter-and-intermolecular-forces/states-of-matter/v/specific-heat-heat-of-fusion-and-vaporization?utm_source=YT&utm;_medium=Desc&utm;_campaign=chemistry Missed the previous lesson? https://www.khanacademy.org/science/chemistry/states-of-matter-and-intermolecular-forces/states-of-matter/v/states-of-matter-follow-up?utm_source=YT&utm;_medium=Desc&utm;_campaign=chemistry Chemistry on Khan Academy: Did you know that everything is made out of chemicals? Chemistry is the study of matter: its composition, properties, and reactivity...

One of the methods of joining two HDPE pipe lengths together is through heat fusion. The above video shows the process of HDPE welding though Heat fusion method in intricate detail.

使用Cam1 https://goo.gl/Y4EiUV Cam2 http://goo.gl/AA0h6V Cam3 http://goo.gl/ekGJo7 Lens https://goo.gl/LdOSf8 Gimbal https://goo.gl/W2GX3H 2 http://goo.gl/LE2zy6 (LUMIX DC-GH5 HC-VX980M FDR-X3000 M.ZUIKO DIGITAL ED 7-14mm F2.8 PRO Zhiyun Crane & Z1 Smooth C &more;)個人趣味の撮影ですが、年100万円前後使って撮影してるので、今年より撮影依頼される場合は1案件あたりの実費相当5,000円~の経費負担願います。SNS・メールに連絡下さい。撮影活動の維持存続にご理解ご協力願います。 連絡先:TW https://twitter.com/nekomon_KENN_39 FB https://www.facebook.com/nekomon1/ nekomon channelオフィシャル猫グッズ:https://www.ttrinity.jp/shop/nekomon/ I spend much money for shooting,but almost shooting without sponsor,please help my channel activity. movie Q&A; , DANCE EVENT report , etc:http://fanblogs.jp/dancemovie/category_5/ 撮影経費と負担額目安:http://fanblogs.jp/dancemovie/archive/145/0 ダンス動画チャンネル メイン DANCE MOVIE channel (Main contents ...


Physics





This video shows you how to do Specific Heat Problems, along with Heat of Fusion and Heat of Vaporization.

4/26/16

A brief podcast on what specific heat i and how to solve basic problems involving specific heat , heat of vaporization and heat of fusion. The video starts with heat and temperature, heating and cooling curves, and where potential and kinetic energy can be found on the heat/cool curve.


This physics video tutorial explains how to solve problems associated with the latent heat of fusion of ice and the latent heat of vaporization of ice. It contains problems associated with specific heat capacity, calorimetry, and heat transfer problems. It discusses the heating curve of water, the increase of kinetic energy during a temperature change process and the increase in potential energy during the phase change process. This video contains plenty of examples and heat transfer practice problems. It discusses phase changes such as freezing, melting, vaporization, condensation and the heat exchange that occurs during those times.

Machining W1 and O1 Tool Steel on the Tormach, then heat treated, then TESTING how hard we got it! Heat Treat Oven: http://amzn.to/2jwNeLR Heat Treat Book: http://amzn.to/2iXGxRO Download Fusion File (Patreon): http://bit.ly/2j3MgCn PTC Hardness Tester: http://amzn.to/2jbHRhw Subscribe For More: http://bit.ly/22CjJoK Patreon: http://bit.ly/NYCpatreon Music copyrighted by John Saunders


































Hate division!
Victims of war - refugees from peace
The quest for reconciliation is in vain
A ruined land - a resisting population
Their future's crushed by false expectations
Hate division!
A valuable culture nearly eliminated
What was strong is now divided by hate
Armed revolution - massacre and slaughter
There's no solution in war, only suffering

