CB-13
From Wikipedia, the free encyclopedia
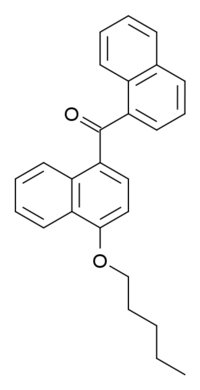 |
|
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| CAS Number | 432047-72-8 |
| PubChem | CID 9799417 |
| ChemSpider | 7975182 |
| ChEMBL | CHEMBL244403 |
| Chemical and physical data | |
|
Systematic (IUPAC) name: naphthalen-1-yl-(4-pentyloxynaphthalen-1-yl)methanone
|
|
| 3D model (Jmol) | Interactive image |
| Formula | C26H24O2 |
| Molar mass | 368.467 g/mol |
|
|
|
|
| (verify) | |
CB-13 (CRA13, SAB-378)[1] is a cannabinoid drug, which acts as a potent agonist at both the CB1 and CB2 receptors, but has poor blood–brain barrier penetration, and so produces only peripheral effects at low doses, with symptoms of central effects such as catalepsy only appearing at much higher dose ranges. It has antihyperalgesic properties in animal studies,[2] and has progressed to preliminary human trials.[3]
Legal Status[edit]
As of October 2015 CB-13 is a controlled substance in China.[4]
See also[edit]
References[edit]
- ^ Cluny, N. L.; Keenan, C. M.; Duncan, M.; Fox, A.; Lutz, B.; Sharkey, K. A. (2010). "Naphthalen-1-yl-(4-pentyloxynaphthalen-1-yl)methanone (SAB378), a Peripherally Restricted Cannabinoid CB1/CB2 Receptor Agonist, Inhibits Gastrointestinal Motility but Has No Effect on Experimental Colitis in Mice". Journal of Pharmacology and Experimental Therapeutics. 334 (3): 973–80. doi:10.1124/jpet.110.169946. PMID 20571060.
- ^ Dziadulewicz, E. K.; Bevan, S. J.; Brain, C. T.; Coote, P. R.; Culshaw, A. J.; Davis, A. J.; Edwards, L. J.; Fisher, A. J.; Fox, A. J.; Gentry, C.; Groarke, A.; Hart, T. W.; Huber, W.; James, I. F.; Kesingland, A.; La Vecchia, L.; Loong, Y.; Lyothier, I.; McNair, K.; O'Farrell, C.; Peacock, M.; Portmann, R.; Schopfer, U.; Yaqoob, M.; Zadrobilek, J. (2007). "Naphthalen-1-yl-(4-pentyloxynaphthalen-1-yl)methanone: A Potent, Orally Bioavailable Human CB1/CB2Dual Agonist with Antihyperalgesic Properties and Restricted Central Nervous System Penetration". Journal of Medicinal Chemistry. 50 (16): 3851–3856. doi:10.1021/jm070317a. PMID 17630726.
- ^ Gardin A, Kucher K, Kiese B, Appel-Dingemanse S (April 2009). "Cannabinoid receptor agonist 13, a novel cannabinoid agonist: first in human pharmacokinetics and safety". Drug Metabolism and Disposition: the Biological Fate of Chemicals. 37 (4): 827–33. doi:10.1124/dmd.108.024000. PMID 19144772.
- ^ "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in Chinese). China Food and Drug Administration. 27 September 2015. Retrieved 1 October 2015.
| This cannabinoid related article is a stub. You can help Wikipedia by expanding it. |