- published: 14 Jan 2010
- views: 13615
-
remove the playlistSulfur Dioxide
- remove the playlistSulfur Dioxide
- published: 16 Jul 2015
- views: 1003
- published: 11 Sep 2014
- views: 115560
- published: 12 Jun 2015
- views: 1069
- published: 07 May 2013
- views: 12612
- published: 06 Jun 2013
- views: 8842
- published: 14 Nov 2011
- views: 15946

Sulfur dioxide (also sulphur dioxide) is the chemical compound with the formula SO2. It is a poisonous gas with a pungent, irritating smell, that is released by volcanoes and in various industrial processes. Since coal and petroleum often contain sulfur compounds, their combustion generates sulfur dioxide unless the sulfur compounds are removed before burning the fuel. Further oxidation of SO2, usually in the presence of a catalyst such as NO2, forms H2SO4, and thus acid rain. Sulfur dioxide emissions are also a precursor to particulates in the atmosphere. Both of these impacts are cause for concern over the environmental impact of these fuels.
SO2 is a bent molecule with C2vsymmetry point group. In terms of electron-counting formalism, the sulfur atom has an oxidation state of +4 and a formal charge of 0. It is surrounded by 5 electron pairs and can be described as a hypervalent molecule. From the perspective of molecular orbital theory, most of these valence electrons are engaged in S–O bonding.
This article is licensed under the Creative Commons Attribution-ShareAlike 3.0 Unported License, which means that you can copy and modify it as long as the entire work (including additions) remains under this license.
- Loading...

-
 6:37
6:37Are You Poisoning Yourself With Sulfur Dioxide in dried fruits
Are You Poisoning Yourself With Sulfur Dioxide in dried fruitsAre You Poisoning Yourself With Sulfur Dioxide in dried fruits
My Central Channel: http://www.MarcusG.tv My facebook http://www.facebook.com/AromaThymeBistro Twitter for my restaurant: http://twitter.com/Aroma_Thyme My personal Twitter: http://twitter.com/healthychefdude My Restaurant website: http://www.aromathymebistro.com/ My restaurant YouTube channel: http://www.youtube.com/user/AromaThyme My wine & beer YouTube channel: http://www.youtube.com/user/chefhbeer Marcus Guiliano, Chef and Owner of Aroma Thyme Bistro located in Ellenville, NY in the scenic Hudson Valley. Aroma Thyme is know for their "stealth health"(Zagat). The Bistro has an award-winning wine list from Wine Spectator Magazine and over 200 beers. Aroma Thyme is certified green by The Green Restaurant Association. Support Hudson Valley Restaurants. -
 2:02
2:02Sources and effects of sulfur dioxide
Sources and effects of sulfur dioxide -
 4:08
4:08Sulfur Dioxide & Exposure Concerns
Sulfur Dioxide & Exposure ConcernsSulfur Dioxide & Exposure Concerns
Sulfur dioxide (SO2) is one of a group of highly reactive gasses known as oxides of sulfur. It is a colorless gas with a pungent and suffocating odor. It is a common air pollutant found in many parts of the world. Much of the sulfur dioxide in the air comes from the burning of coal and oil at electric power plants. Other sources of sulfur dioxide come from industrial facilities that use coal or oil, petroleum refineries, cement manufacturing, metal mining and processing, paper pulp manufacturing and copper smelting. Trains, large ships and some diesel equipment may burn high sulfur fuels which also contributes to sulfur dioxide in the air. Sulfur dioxide has also been used as a food preservative and for food processing; as a disinfectant; for bleaching flour, fruit, grain, wood pulp, wool, textile fibers, wicker, gelatin and glue; and for making other chemicals. It is also used for wastewater treatment. Sulfur dioxide and nitrogen oxides can react with precipitation, oxygen and other substances in the atmosphere to form acid rain. People can be exposed to sulfur dioxide outdoors by breathing polluted air. This is more likely to occur in the summer, when the sun and hot temperatures react with pollution to form smog. Natural pollution sources, such as plant decay and volcanoes can also expose people to this gas. People who live near or work in facilities that utilize sulfur dioxide or produce it as a by-product may also be exposed. According to the U.S. National Library of Medicine, “Breathing sulfur dioxide can irritate the nose, throat, and lungs, and cause coughing and shortness of breath. Short-term exposure to sulfur dioxide can cause stomach pain, menstrual disorders, watery eyes, inhibition of thyroid function, loss of smell, headache, nausea, vomiting, fever, convulsions, and dizziness.” They also report, “Short-term exposure to high levels of sulfur dioxide in the air can be life-threatening by causing breathing difficulties and obstructing airways, especially for people with lung disease. Long-term exposure to persistent levels of sulfur dioxide can cause chronic bronchitis, emphysema, and respiratory illness. It can also aggravate existing heart disease.” These are just a few things to know about sulfur dioxide, exposure risks and potential health concerns. To learn more about this or other indoor and outdoor air quality, health and safety, occupational or environmental issues, please visit the websites shown below. Clark Seif Clark http://www.csceng.com EMSL Analytical, Inc. http://www.emsl.com Indoor Environmental Consultants, Inc. http://www.iecinc.net LA Testing http://www.latesting.com Zimmetry Environmental http://www.zimmetry.com Healthy Indoors Magazine http://www.iaq.net Hudson Douglas Public Adjusters http://HudsonDouglasPublicAdjusters.com -
 4:16
4:16Making Sulfur Dioxide
Making Sulfur DioxideMaking Sulfur Dioxide
Making Sulfur Dioxide -
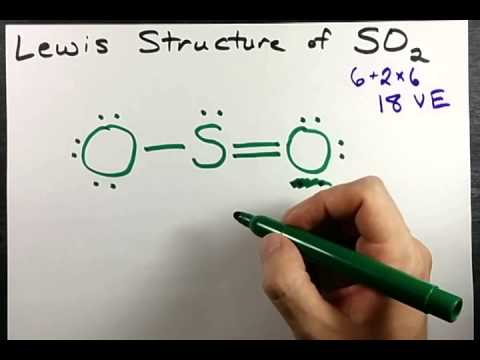 4:59
4:59Lewis Structure of SO2 (sulfur dioxide)
Lewis Structure of SO2 (sulfur dioxide)Lewis Structure of SO2 (sulfur dioxide)
How to draw the Lewis Structure of SO2 - with explanation Check me out: http://www.chemistnate.com -
 4:24
4:24Sulfur Dioxide
Sulfur DioxideSulfur Dioxide
-
 2:29
2:29How to make Sulfur dioxide gas
How to make Sulfur dioxide gasHow to make Sulfur dioxide gas
Sulfur dioxide is very toxic gas. so do this experiments outdoor. First put some sodium sulphite into flask. and put some dil. hydrochloric acid. and it starts to bubbling SO2 gas. you put wet flower into SO2 gas you can see the color will lose. -
 0:58
0:58SO2 + H2O Sulfur dioxide and water המסת גופרית דו חמצנית במים
SO2 + H2O Sulfur dioxide and water המסת גופרית דו חמצנית במיםSO2 + H2O Sulfur dioxide and water המסת גופרית דו חמצנית במים
S8 + O2 then SO2 + H2O Sulfur dioxide and water שריפה של גופרית, והמסת הגז דו חמצנית שנוצר במים צפייה בסמארטפונים והסבר מלא בקישור: http://goo.gl/GdpdN הסרט הוא חלק מסדרה של ניסויים אינטראקטיבים בנושא "חומצה בסיס", הסידרה כוללת את כל תגובות החומצה-בסיס הנלמדות במסגרת הבגרות בכימיה בתיכון בישראל -- ליחצו על ההערות המופיעות במהלך הסרט כדי לעבור בין הסרטים והניסויים המצולמים רעיון: ד"ר אבי סאייג - מכון דוידסון לחינוך מדעי, מכון ויצמן למדע פיתוח והפקה: ד"ר אבי סאייג, ד"ר מלכה יאיון, מרכז מורי הכימיה הארצי, המחלקה להוראת המדעים, מכון ויצמן למדע מציגים: ד"ר אבי סאייג, שלי רפּ - המחלקה להוראת המדעים, מכון ויצמן למדע צילום: שגיא בר און עריכה: צילה ביטרמן מוזיקה: Amazing Plan; Hustle. Kevin MacLeod - Creative Commons license, http://incompetech.com/m/c/royalty-free/ -
 1:56
1:56Sulphur Dioxide forms an acid | Acids & Bases | Chemistry
Sulphur Dioxide forms an acid | Acids & Bases | ChemistrySulphur Dioxide forms an acid | Acids & Bases | Chemistry
If we leave a glass of water in the open air for a few days, the water will turn it slightly acidic. This happens because the gaseous carbon dioxide in air dissolves in water to form carbonic acid. Like carbon dioxide, which is an oxide of a non-metallic element (Carbon), this property is also true generally true for oxides of other non-metals. The video demonstrates the heating of sulphur in a test tube until it oxidizes (burns) and releases sulphur dioxide smoke. Sulphur dioxide does not affect red litmus but changes the colour of wet blue litmus paper to red, showing us that sulphur dioxide gas is acidic. Sulphuric acid is produced by dissolving sulphur dioxide in water. Many industrial processes produce sulphur dioxide gas as a pollutant and when this gas dissolves in rain water, we get acid rain which is harmful to the biosphere. -
 4:52
4:52How to make some useful gases (Hydrogen, Sulfur Dioxide, Carbon Dioxide and more)
How to make some useful gases (Hydrogen, Sulfur Dioxide, Carbon Dioxide and more)How to make some useful gases (Hydrogen, Sulfur Dioxide, Carbon Dioxide and more)
In this epsode I show how to make some gases that allow for interesting chemistry. I only show one as it would become repetitive but all are listed with instructions at the end. My website: thechemlife.com My facebook page: http://www.facebook.com/pages/TheChemLife/128028337270470 My twitter: https://twitter.com/#!/thechemlife
- Acid rain
- Acid Rain Program
- Anhydrite
- Antibiotic
- Antimicrobial
- Antioxidant
- Apricot
- Beilstein database
- Bleach
- Boiling point
- Bond order
- Calcium
- Calcium oxide
- Calcium silicate
- Calcium sulfate
- Calcium sulfite
- CAS registry number
- Cement
- ChEBI
- Cheletropic reaction
- ChEMBL
- Chemical compound
- Chemical formula
- ChemSpider
- China
- Chloride
- Chlorofluorocarbon
- Cinnabar
- Claus process
- Coal
- Coke (fuel)
- Contact process
- Cork taint
- CRC Press
- Decomposition
- Density
- Diazonium salt
- Diene
- Dipole
- Dried fruit
- E number
- Electron counting
- Electron pair
- Formal charge
- Gas
- Gmelin database
- Halemaumau Crater
- Hapticity
- Heat of evaporation
- Hering–Breuer reflex
- Hydrogen sulfide
- Hypervalent molecule
- IDLH
- Jmol
- KEGG
- Ligand
- List of R-phrases
- List of S-phrases
- Magnesium oxide
- Median lethal dose
- Melting point
- Molar mass
- Molasses
- Molecular geometry
- NFPA 704
- NIOSH
- Nitrogen dioxide
- Oil refineries
- Oleum
- Organic Syntheses
- Organic synthesis
- Oxidation
- Oxidation state
- Oxide
- Oxidising agent
- Oxygen
- Ozone
- Paper
- Particulates
- Petroleum
- Power plant
- Preservative
- Preterm birth
- PubChem
- PubMed Identifier
- Pyrite
- R34 Causes burns
- Redox
- Reducing agent
- Refrigerant
- Rosé
- RTECS
- Selenium dioxide
- Short ton
- Sodium bisulfite
- Sodium metabisulfite
- Solubility
- Space group
- Special ComparePages
- Sphalerite
- Standard state
- Stretford process
- Sulfite
- Sulfur
- Sulfur dioxide
- Sulfur monoxide
- Sulfur trioxide
- Sulfur-iodine cycle
- Sulfuric acid
- Sulfurous acid
- Sulfuryl chloride
- Swarf
- Symmetry point group
- Systematic review
- Tellurium dioxide
- UN number
- United States
- Valence electron
- Vapor pressure
- Viscosity
- Volcano
- Water
- Wikipedia Link rot
- Winemaking
-

Are You Poisoning Yourself With Sulfur Dioxide in dried fruits
My Central Channel: http://www.MarcusG.tv My facebook http://www.facebook.com/AromaThymeBistro Twitter for my restaurant: http://twitter.com/Aroma_Thyme My personal Twitter: http://twitter.com/healthychefdude My Restaurant website: http://www.aromathymebistro.com/ My restaurant YouTube channel: http://www.youtube.com/user/AromaThyme My wine & beer YouTube channel: http://www.youtube.com/user/chefhbeer Marcus Guiliano, Chef and Owner of Aroma Thyme Bistro located in Ellenville, NY in the scenic Hudson Valley. Aroma Thyme is know for their "stealth health"(Zagat). The Bistro has an award-winning wine list from Wine Spectator Magazine and over 200 beers. Aroma Thyme is certified green by The Green Restaurant Association. Support Hudson Valley Restaurants. -

-

Sulfur Dioxide & Exposure Concerns
Sulfur dioxide (SO2) is one of a group of highly reactive gasses known as oxides of sulfur. It is a colorless gas with a pungent and suffocating odor. It is a common air pollutant found in many parts of the world. Much of the sulfur dioxide in the air comes from the burning of coal and oil at electric power plants. Other sources of sulfur dioxide come from industrial facilities that use coal or oil, petroleum refineries, cement manufacturing, metal mining and processing, paper pulp manufacturing and copper smelting. Trains, large ships and some diesel equipment may burn high sulfur fuels which also contributes to sulfur dioxide in the air. Sulfur dioxide has also been used as a food preservative and for food processing; as a disinfectant; for bleaching flour, fruit, grain, wood pulp, ... -

Making Sulfur Dioxide
Making Sulfur Dioxide -

Lewis Structure of SO2 (sulfur dioxide)
How to draw the Lewis Structure of SO2 - with explanation Check me out: http://www.chemistnate.com -

Sulfur Dioxide
-

How to make Sulfur dioxide gas
Sulfur dioxide is very toxic gas. so do this experiments outdoor. First put some sodium sulphite into flask. and put some dil. hydrochloric acid. and it starts to bubbling SO2 gas. you put wet flower into SO2 gas you can see the color will lose. -

SO2 + H2O Sulfur dioxide and water המסת גופרית דו חמצנית במים
S8 + O2 then SO2 + H2O Sulfur dioxide and water שריפה של גופרית, והמסת הגז דו חמצנית שנוצר במים צפייה בסמארטפונים והסבר מלא בקישור: http://goo.gl/GdpdN הסרט הוא חלק מסדרה של ניסויים אינטראקטיבים בנושא "חומצה בסיס", הסידרה כוללת את כל תגובות החומצה-בסיס הנלמדות במסגרת הבגרות בכימיה בתיכון בישראל -- ליחצו על ההערות המופיעות במהלך הסרט כדי לעבור בין הסרטים והניסויים המצולמים רעיון: ד"ר אבי סאייג - מכון דוידסון לחינוך מדעי, מכון ויצמן למדע פיתוח והפקה: ד"ר אבי סאייג, ד"ר מלכה יאיון, מרכז מורי הכימיה הארצי, המחלקה להוראת המדעים, מכון ויצמן למדע מציגים: ד"ר אבי סאייג, שלי רפּ - המחלקה להוראת המדעים, מכון ויצמן למדע צילום: שגיא בר און עריכה: צילה ביטרמן מוזיקה: Amazing Plan; Hustle. Kevin MacLeod - Creative Commons license, http://incompetech.com/m/c/royalty-free/ -

Sulphur Dioxide forms an acid | Acids & Bases | Chemistry
If we leave a glass of water in the open air for a few days, the water will turn it slightly acidic. This happens because the gaseous carbon dioxide in air dissolves in water to form carbonic acid. Like carbon dioxide, which is an oxide of a non-metallic element (Carbon), this property is also true generally true for oxides of other non-metals. The video demonstrates the heating of sulphur in a test tube until it oxidizes (burns) and releases sulphur dioxide smoke. Sulphur dioxide does not affect red litmus but changes the colour of wet blue litmus paper to red, showing us that sulphur dioxide gas is acidic. Sulphuric acid is produced by dissolving sulphur dioxide in water. Many industrial processes produce sulphur dioxide gas as a pollutant and when this gas dissolves in rain water, we ge... -

How to make some useful gases (Hydrogen, Sulfur Dioxide, Carbon Dioxide and more)
In this epsode I show how to make some gases that allow for interesting chemistry. I only show one as it would become repetitive but all are listed with instructions at the end. My website: thechemlife.com My facebook page: http://www.facebook.com/pages/TheChemLife/128028337270470 My twitter: https://twitter.com/#!/thechemlife
Are You Poisoning Yourself With Sulfur Dioxide in dried fruits
- Order: Reorder
- Duration: 6:37
- Updated: 14 Jan 2010
- views: 13615
- published: 14 Jan 2010
- views: 13615
Sources and effects of sulfur dioxide
- Order: Reorder
- Duration: 2:02
- Updated: 11 Jun 2014
- views: 3074
Sulfur Dioxide & Exposure Concerns
- Order: Reorder
- Duration: 4:08
- Updated: 16 Jul 2015
- views: 1003
- published: 16 Jul 2015
- views: 1003
Making Sulfur Dioxide
- Order: Reorder
- Duration: 4:16
- Updated: 26 Feb 2013
- views: 6776
Lewis Structure of SO2 (sulfur dioxide)
- Order: Reorder
- Duration: 4:59
- Updated: 11 Sep 2014
- views: 115560
- published: 11 Sep 2014
- views: 115560
Sulfur Dioxide
- Order: Reorder
- Duration: 4:24
- Updated: 30 Jan 2012
- views: 6988
- published: 30 Jan 2012
- views: 6988
How to make Sulfur dioxide gas
- Order: Reorder
- Duration: 2:29
- Updated: 12 Jun 2015
- views: 1069
- published: 12 Jun 2015
- views: 1069
SO2 + H2O Sulfur dioxide and water המסת גופרית דו חמצנית במים
- Order: Reorder
- Duration: 0:58
- Updated: 07 May 2013
- views: 12612
- published: 07 May 2013
- views: 12612
Sulphur Dioxide forms an acid | Acids & Bases | Chemistry
- Order: Reorder
- Duration: 1:56
- Updated: 06 Jun 2013
- views: 8842
- published: 06 Jun 2013
- views: 8842
How to make some useful gases (Hydrogen, Sulfur Dioxide, Carbon Dioxide and more)
- Order: Reorder
- Duration: 4:52
- Updated: 14 Nov 2011
- views: 15946
- published: 14 Nov 2011
- views: 15946
- Playlist
- Chat
- Playlist
- Chat

Are You Poisoning Yourself With Sulfur Dioxide in dried fruits
- Report rights infringement
- published: 14 Jan 2010
- views: 13615

Sources and effects of sulfur dioxide
- Report rights infringement
- published: 11 Jun 2014
- views: 3074

Sulfur Dioxide & Exposure Concerns
- Report rights infringement
- published: 16 Jul 2015
- views: 1003

Making Sulfur Dioxide
- Report rights infringement
- published: 26 Feb 2013
- views: 6776

Lewis Structure of SO2 (sulfur dioxide)
- Report rights infringement
- published: 11 Sep 2014
- views: 115560

Sulfur Dioxide
- Report rights infringement
- published: 30 Jan 2012
- views: 6988

How to make Sulfur dioxide gas
- Report rights infringement
- published: 12 Jun 2015
- views: 1069

SO2 + H2O Sulfur dioxide and water המסת גופרית דו חמצנית במים
- Report rights infringement
- published: 07 May 2013
- views: 12612

Sulphur Dioxide forms an acid | Acids & Bases | Chemistry
- Report rights infringement
- published: 06 Jun 2013
- views: 8842

How to make some useful gases (Hydrogen, Sulfur Dioxide, Carbon Dioxide and more)
- Report rights infringement
- published: 14 Nov 2011
- views: 15946
Archaeologists Find Skeletons, Gold Coins In Buried Pompeii Shop
Edit WorldNews.com 24 Jun 2016Musician David Byrne Pens Stinging Gun-Control Essay Aimed At US
Edit WorldNews.com 24 Jun 2016Brexit: David Cameron resigns as prime minister
Edit The Irish Times 24 Jun 2016Investigators: China Still Harvesting Human Organs on Huge Scale
Edit Voa News 24 Jun 2016San Andreas study confirms danger of fault
Edit WPTV 24 Jun 2016China green finance surges in 2015 (The Central People's Government of the People's Republic of China)
Edit Public Technologies 24 Jun 2016One of the five shops of Nickel Plant, the Sinter Shop was shut down in Norilsk. (OJSC MMC Norilsk Nickel)
Edit Public Technologies 23 Jun 2016Wisconsin Air Quality Improvement Project Produces Cleaner Energy (Stanley Consultants Inc)
Edit Public Technologies 22 Jun 2016Making blue skies over Jing-Jin-Ji last
Edit China Daily 21 Jun 2016Outotec to revamp a copper smelter and sulfuric acid plant in South America (Outotec Oyj)
Edit Public Technologies 20 Jun 2016Beijing PM2.5 density down by 19.3 percent Jan.-May (Beijing Municipal Government)
Edit Public Technologies 17 Jun 2016Beilun becomes provincial low-carbon pilot (Ningbo Municipal Government)
Edit Public Technologies 17 Jun 2016Brazil’s pizza habit has a surprisingly high environmental cost
Edit Vox 17 Jun 2016NASA Awards Grants for University Research and Development Programs (NASA - The National Aeronautics and Space Administration)
Edit Public Technologies 16 Jun 2016Killer surf and Kilauea hotspots, on Hawaii’s Big Island
Edit Atlanta Journal 16 Jun 20166/14/2016 - American wind power celebrates Global Wind Day with two-thirds-off sale (AWEA - American Wind Energy Association)
Edit Public Technologies 15 Jun 2016- 1
- 2
- 3
- 4
- 5
- Next page »







