- published: 15 Jun 2015
- views: 262444
-
remove the playlistOxidation State
-
remove the playlistLatest Videos
-
remove the playlistLongest Videos
- remove the playlistOxidation State
- remove the playlistLatest Videos
- remove the playlistLongest Videos
- published: 09 Jan 2014
- views: 178529
- published: 27 Mar 2012
- views: 543625
- published: 28 Mar 2014
- views: 36590
- published: 17 Jun 2015
- views: 111717
- published: 23 Apr 2013
- views: 1161912
- published: 27 Nov 2011
- views: 117447
- published: 05 Oct 2015
- views: 5590
- published: 08 Jan 2014
- views: 139815

Oxidation state
The oxidation state, often called the oxidation number, is an indicator of the degree of oxidation (loss of electrons) of an atom in a chemical compound. Conceptually, the oxidation state, which may be positive, negative or zero, is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic, with no covalent component. This is never exactly true for real bonds.
The term "oxidation" was first used by Lavoisier to mean reaction of a substance with oxygen. Much later, it was realized that the substance, upon being oxidized, loses electrons, and the use of the term "oxidation" was extended to include other reactions in which electrons are lost.
Oxidation states are typically represented by integers. In some cases, the average oxidation state of an element is a fraction, such as 8/3 for iron in magnetite (Fe
3O
4). The highest known oxidation state is reported to be +9 in the iridium tetroxide cation (IrO+
4), while the lowest known oxidation state is −5 for boron, gallium, indium, and thallium in various intermetallic compounds. The possibility of +10 oxidation states in platinum group elements, especially platinum(X), has been discussed by Kiselev and Tretiyakov.
This article is licensed under the Creative Commons Attribution-ShareAlike 3.0 Unported License, which means that you can copy and modify it as long as the entire work (including additions) remains under this license.

Khan Academy
Khan Academy is a non-profit educational organization created in 2006 by educator Salman Khan with the aim of providing a free, world-class education for anyone, anywhere. The organization produces short lectures in the form of YouTube videos. In addition to micro lectures, the organization's website features practice exercises and tools for educators. All resources are available for free to anyone around the world. The main language of the website is English, but the content is also available in other languages.
History
The founder of the organization, Salman Khan, was born in New Orleans, Louisiana, United States to immigrant parents from Bangladesh and India. After earning three degrees from the Massachusetts Institute of Technology (a BS in mathematics, a BS in electrical engineering and computer science, and an MEng in electrical engineering and computer science), he pursued an MBA from Harvard Business School.
In late 2004, Khan began tutoring his cousin Nadia who needed help with math using Yahoo!'s Doodle notepad.When other relatives and friends sought similar help, he decided that it would be more practical to distribute the tutorials on YouTube. The videos' popularity and the testimonials of appreciative students prompted Khan to quit his job in finance as a hedge fund analyst at Connective Capital Management in 2009, and focus on the tutorials (then released under the moniker "Khan Academy") full-time.
This article is licensed under the Creative Commons Attribution-ShareAlike 3.0 Unported License, which means that you can copy and modify it as long as the entire work (including additions) remains under this license.

Transition metal
In chemistry, the term transition metal (or transition element) has three possible meanings:
English chemist Charles Bury (1890-1968) first used the word transition in this context in 1921, when he referred to a transition series of elements during the change of an inner layer of electrons (for example n=3 in the 4th row of the periodic table) from a stable group of 8 to one of 18, or from 18 to 32. These elements are now known as the d-block.
This article is licensed under the Creative Commons Attribution-ShareAlike 3.0 Unported License, which means that you can copy and modify it as long as the entire work (including additions) remains under this license.
- Loading...

-
 13:26
13:26How to Calculate Oxidation Numbers Introduction
How to Calculate Oxidation Numbers IntroductionHow to Calculate Oxidation Numbers Introduction
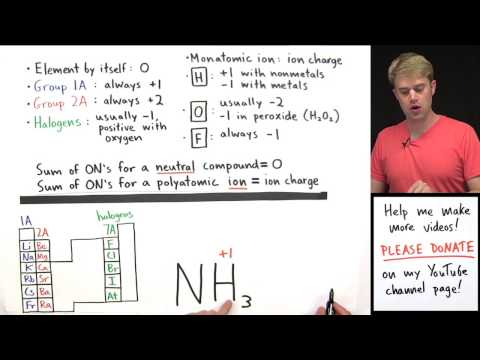
We'll learn how to determine the oxidation numbers or oxidation states for a the elements in a chemical compound. The oxidation numbers tell us how electrons are divided up or shared between atoms in a chemical compound. The oxidation numbers also tell us how electrons move in an oxidation reduction (redox) reaction. There are a set a rules that we use to determine oxidation number. Group 1A elements (alkalai metals) always have an oxidation of +1. Group 2A elements (alkaline earth metals) always have an oxidation number of +2. Elements on their own have an oxidation number of 0, and monatomic ions have an oxidation number that is equal to the ionic charge. -
 4:27
4:27Practice determining oxidation states | Chemistry | Khan Academy
Practice determining oxidation states | Chemistry | Khan AcademyPractice determining oxidation states | Chemistry | Khan Academy
"Determining oxidation numbers in magnesium oxide and magnesium hydroxide. Watch the next lesson: https://www.khanacademy.org/science/chemistry/oxidation-reduction/redox-oxidation-reduction/v/unusual-oxygen-oxidation-states?utm_source=YT&utm;_medium=Desc&utm;_campaign=chemistry Missed the previous lesson? https://www.khanacademy.org/science/chemistry/oxidation-reduction/redox-oxidation-reduction/v/oxidation-state-trends-in-periodic-table?utm_source=YT&utm;_medium=Desc&utm;_campaign=chemistry Chemistry on Khan Academy: Did you know that everything is made out of chemicals? Chemistry is the study of matter: its composition, properties, and reactivity. This material roughly covers a first-year high school or college course, and a good understanding of algebra is helpful. About Khan Academy: Khan Academy offers practice exercises, instructional videos, and a personalized learning dashboard that empower learners to study at their own pace in and outside of the classroom. We tackle math, science, computer programming, history, art history, economics, and more. Our math missions guide learners from kindergarten to calculus using state-of-the-art, adaptive technology that identifies strengths and learning gaps. We've also partnered with institutions like NASA, The Museum of Modern Art, The California Academy of Sciences, and MIT to offer specialized content. For free. For everyone. Forever. #YouCanLearnAnything Subscribe to Khan Academy’s Chemistry channel: https://www.youtube.com/channel/UCyEot66LrwWFEMONvrIBh3A?sub_confirmation=1 Subscribe to Khan Academy: https://www.youtube.com/subscription_center?add_user=khanacademy -
 9:37
9:37How to Figure out Oxidation Numbers
How to Figure out Oxidation NumbersHow to Figure out Oxidation Numbers
How to assign oxidation numbers to the atoms in a molecule. 1. Elements have oxidation number = 0 2. Hydrogen's always +1 (except in "hydrides") 3. Oxygen's always -2 (except in "peroxides") 4. Other atoms get the charge they prefer, as long as the sum of oxidation numbers for all atoms = the total charge on the atom. -
 17:39
17:39Introduction to Oxidation States
Introduction to Oxidation States -
 30:38
30:384.1 Introduction to Oxidation state
4.1 Introduction to Oxidation state4.1 Introduction to Oxidation state
In this video I have taught what is oxidation state and what the most easy method to find the oxidation state. As knowledge of oxidation state is needed throughout the chemistry,this is a must watch video. Enjoy it. -
 15:25
15:25How to Calculate Oxidation Number Practice Problems
How to Calculate Oxidation Number Practice ProblemsHow to Calculate Oxidation Number Practice Problems
Many practice problems for how to calculate and determine oxidation numbers, often referred to as oxidation states. To figure out oxidation numbers for elements in a compound, we have to look on the periodic table and consult a list of rules. For neutral compounds, the oxidation numbers add up to zero. For polyatomic ions, the oxidation numbers add up to charge of the ion. -
 11:13
11:13Redox Reactions: Crash Course Chemistry #10
Redox Reactions: Crash Course Chemistry #10Redox Reactions: Crash Course Chemistry #10
All the magic that we know is in the transfer of electrons. Reduction (gaining electrons) and oxidation (the loss of electrons) combine to form Redox chemistry, which contains the majority of chemical reactions. As electrons jump from atom to atom, they carry energy with them, and that transfer of energy is what makes all life on earth possible. **Special Thanks to Matt Young at the University of Montana (Geosciences Department, Environmental Biogeochemistry Lab) who helped with the chemical demonstrations.** Oxidation 1:42 Reduction 1:03 Oxidation Numbers 3:29 Redox Reactions 5:59 Oxidation Reactions 6:28 Balancing Oxidation Reactions 7:18 Also thank you to the following chemistry teachers for assistance: James Sarbinoff Rachel Wentz Edi González Lucas Moore Chris Conley Addie Clark Julia Rosinski Want to find Crash Course elsewhere on the internet? Facebook - http://www.facebook.com/YouTubeCrashC... Twitter - http://www.twitter.com/TheCrashCourse Tumblr - http://thecrashcourse.tumblr.com Support CrashCourse on Subbable: http://subbable.com/crashcourse -
 6:00
6:00Assigning Oxidation Numbers - Chemistry Tutorial
Assigning Oxidation Numbers - Chemistry TutorialAssigning Oxidation Numbers - Chemistry Tutorial
This chemistry tutorial discusses how to assign oxidation numbers and includes examples of how to determine the oxidation numbers in a compound following some simple rules. https://www.thechemistrysolution.com -
 19:46
19:46Oxidation Numbers & States Explained - Rules, Polyatomic Ions, Compounds, & Transition Metals
Oxidation Numbers & States Explained - Rules, Polyatomic Ions, Compounds, & Transition MetalsOxidation Numbers & States Explained - Rules, Polyatomic Ions, Compounds, & Transition Metals
This chemistry video tutorial shows you how to determine the oxidation state or oxidation number of an element in a compound or a transition metal within a polyatomic ion. This video contains plenty of examples and practice problems for you to work on. Here is a list of topics: 1. Assigning Oxidation Numbers To Elements in Compounds 2. Determining The Oxidation States of Transition Metals 3. Calculating The Oxidation Number of a Element in a polyatomic ion 4. Oxidation Number Rules - Pure Elements Always Zero 5. Oxidation States - Electronegativity - Fluorine is -1 6. Oxidation Rules - Oxygen Usually is -2 in Oxide and -2 in Peroxide 7. Hydrogen is +1 when bonded to a nonmetal and -1 when bonded to a metal 8. Transition Metals - Multiple Oxidation States -
 8:38
8:38Oxidation state trends in periodic table | Chemistry | Khan Academy
Oxidation state trends in periodic table | Chemistry | Khan AcademyOxidation state trends in periodic table | Chemistry | Khan Academy
Trends in common oxidation states for main group elements. Watch the next lesson: https://www.khanacademy.org/science/chemistry/oxidation-reduction/redox-oxidation-reduction/v/practice-determining-oxidation-states?utm_source=YT&utm;_medium=Desc&utm;_campaign=chemistry Missed the previous lesson? https://www.khanacademy.org/science/chemistry/oxidation-reduction/redox-oxidation-reduction/v/introduction-to-oxidation-and-reduction?utm_source=YT&utm;_medium=Desc&utm;_campaign=chemistry Chemistry on Khan Academy: Did you know that everything is made out of chemicals? Chemistry is the study of matter: its composition, properties, and reactivity. This material roughly covers a first-year high school or college course, and a good understanding of algebra is helpful. About Khan Academy: Khan Academy offers practice exercises, instructional videos, and a personalized learning dashboard that empower learners to study at their own pace in and outside of the classroom. We tackle math, science, computer programming, history, art history, economics, and more. Our math missions guide learners from kindergarten to calculus using state-of-the-art, adaptive technology that identifies strengths and learning gaps. We've also partnered with institutions like NASA, The Museum of Modern Art, The California Academy of Sciences, and MIT to offer specialized content. For free. For everyone. Forever. #YouCanLearnAnything Subscribe to Khan Academy’s Chemistry channel: https://www.youtube.com/channel/UCyEot66LrwWFEMONvrIBh3A?sub_confirmation=1 Subscribe to Khan Academy: https://www.youtube.com/subscription_center?add_user=khanacademy
-

How to Calculate Oxidation Numbers Introduction
We'll learn how to determine the oxidation numbers or oxidation states for a the elements in a chemical compound. The oxidation numbers tell us how electrons are divided up or shared between atoms in a chemical compound. The oxidation numbers also tell us how electrons move in an oxidation reduction (redox) reaction. There are a set a rules that we use to determine oxidation number. Group 1A elements (alkalai metals) always have an oxidation of +1. Group 2A elements (alkaline earth metals) always have an oxidation number of +2. Elements on their own have an oxidation number of 0, and monatomic ions have an oxidation number that is equal to the ionic charge.
published: 15 Jun 2015 -

Practice determining oxidation states | Chemistry | Khan Academy
"Determining oxidation numbers in magnesium oxide and magnesium hydroxide. Watch the next lesson: https://www.khanacademy.org/science/chemistry/oxidation-reduction/redox-oxidation-reduction/v/unusual-oxygen-oxidation-states?utm_source=YT&utm;_medium=Desc&utm;_campaign=chemistry Missed the previous lesson? https://www.khanacademy.org/science/chemistry/oxidation-reduction/redox-oxidation-reduction/v/oxidation-state-trends-in-periodic-table?utm_source=YT&utm;_medium=Desc&utm;_campaign=chemistry Chemistry on Khan Academy: Did you know that everything is made out of chemicals? Chemistry is the study of matter: its composition, properties, and reactivity. This material roughly covers a first-year high school or college course, and a good understanding of algebra is helpful. About Khan Academy: ...
published: 09 Jan 2014 -

How to Figure out Oxidation Numbers
How to assign oxidation numbers to the atoms in a molecule. 1. Elements have oxidation number = 0 2. Hydrogen's always +1 (except in "hydrides") 3. Oxygen's always -2 (except in "peroxides") 4. Other atoms get the charge they prefer, as long as the sum of oxidation numbers for all atoms = the total charge on the atom.
published: 27 Mar 2012 -

-

4.1 Introduction to Oxidation state
In this video I have taught what is oxidation state and what the most easy method to find the oxidation state. As knowledge of oxidation state is needed throughout the chemistry,this is a must watch video. Enjoy it.
published: 28 Mar 2014 -

How to Calculate Oxidation Number Practice Problems
Many practice problems for how to calculate and determine oxidation numbers, often referred to as oxidation states. To figure out oxidation numbers for elements in a compound, we have to look on the periodic table and consult a list of rules. For neutral compounds, the oxidation numbers add up to zero. For polyatomic ions, the oxidation numbers add up to charge of the ion.
published: 17 Jun 2015 -

Redox Reactions: Crash Course Chemistry #10
All the magic that we know is in the transfer of electrons. Reduction (gaining electrons) and oxidation (the loss of electrons) combine to form Redox chemistry, which contains the majority of chemical reactions. As electrons jump from atom to atom, they carry energy with them, and that transfer of energy is what makes all life on earth possible. **Special Thanks to Matt Young at the University of Montana (Geosciences Department, Environmental Biogeochemistry Lab) who helped with the chemical demonstrations.** Oxidation 1:42 Reduction 1:03 Oxidation Numbers 3:29 Redox Reactions 5:59 Oxidation Reactions 6:28 Balancing Oxidation Reactions 7:18 Also thank you to the following chemistry teachers for assistance: James Sarbinoff Rachel Wentz Edi González Lucas Moore Chris Conley Addie Clark J...
published: 23 Apr 2013 -

Assigning Oxidation Numbers - Chemistry Tutorial
This chemistry tutorial discusses how to assign oxidation numbers and includes examples of how to determine the oxidation numbers in a compound following some simple rules. https://www.thechemistrysolution.com
published: 27 Nov 2011 -

Oxidation Numbers & States Explained - Rules, Polyatomic Ions, Compounds, & Transition Metals
This chemistry video tutorial shows you how to determine the oxidation state or oxidation number of an element in a compound or a transition metal within a polyatomic ion. This video contains plenty of examples and practice problems for you to work on. Here is a list of topics: 1. Assigning Oxidation Numbers To Elements in Compounds 2. Determining The Oxidation States of Transition Metals 3. Calculating The Oxidation Number of a Element in a polyatomic ion 4. Oxidation Number Rules - Pure Elements Always Zero 5. Oxidation States - Electronegativity - Fluorine is -1 6. Oxidation Rules - Oxygen Usually is -2 in Oxide and -2 in Peroxide 7. Hydrogen is +1 when bonded to a nonmetal and -1 when bonded to a metal 8. Transition Metals - Multiple Oxidation States
published: 05 Oct 2015 -

Oxidation state trends in periodic table | Chemistry | Khan Academy
Trends in common oxidation states for main group elements. Watch the next lesson: https://www.khanacademy.org/science/chemistry/oxidation-reduction/redox-oxidation-reduction/v/practice-determining-oxidation-states?utm_source=YT&utm;_medium=Desc&utm;_campaign=chemistry Missed the previous lesson? https://www.khanacademy.org/science/chemistry/oxidation-reduction/redox-oxidation-reduction/v/introduction-to-oxidation-and-reduction?utm_source=YT&utm;_medium=Desc&utm;_campaign=chemistry Chemistry on Khan Academy: Did you know that everything is made out of chemicals? Chemistry is the study of matter: its composition, properties, and reactivity. This material roughly covers a first-year high school or college course, and a good understanding of algebra is helpful. About Khan Academy: Khan Academ...
published: 08 Jan 2014
How to Calculate Oxidation Numbers Introduction
- Order: Reorder
- Duration: 13:26
- Updated: 15 Jun 2015
- views: 262444
- published: 15 Jun 2015
- views: 262444
Practice determining oxidation states | Chemistry | Khan Academy
- Order: Reorder
- Duration: 4:27
- Updated: 09 Jan 2014
- views: 178529
- published: 09 Jan 2014
- views: 178529
How to Figure out Oxidation Numbers
- Order: Reorder
- Duration: 9:37
- Updated: 27 Mar 2012
- views: 543625
- published: 27 Mar 2012
- views: 543625
Introduction to Oxidation States
- Order: Reorder
- Duration: 17:39
- Updated: 10 Sep 2009
- views: 366668
4.1 Introduction to Oxidation state
- Order: Reorder
- Duration: 30:38
- Updated: 28 Mar 2014
- views: 36590
- published: 28 Mar 2014
- views: 36590
How to Calculate Oxidation Number Practice Problems
- Order: Reorder
- Duration: 15:25
- Updated: 17 Jun 2015
- views: 111717
- published: 17 Jun 2015
- views: 111717
Redox Reactions: Crash Course Chemistry #10
- Order: Reorder
- Duration: 11:13
- Updated: 23 Apr 2013
- views: 1161912
- published: 23 Apr 2013
- views: 1161912
Assigning Oxidation Numbers - Chemistry Tutorial
- Order: Reorder
- Duration: 6:00
- Updated: 27 Nov 2011
- views: 117447
- published: 27 Nov 2011
- views: 117447
Oxidation Numbers & States Explained - Rules, Polyatomic Ions, Compounds, & Transition Metals
- Order: Reorder
- Duration: 19:46
- Updated: 05 Oct 2015
- views: 5590
- published: 05 Oct 2015
- views: 5590
Oxidation state trends in periodic table | Chemistry | Khan Academy
- Order: Reorder
- Duration: 8:38
- Updated: 08 Jan 2014
- views: 139815
- published: 08 Jan 2014
- views: 139815
-

How to Calculate Oxidation Numbers Introduction
We'll learn how to determine the oxidation numbers or oxidation states for a the elements in a chemical compound. The oxidation numbers tell us how electrons are divided up or shared between atoms in a chemical compound. The oxidation numbers also tell us how electrons move in an oxidation reduction (redox) reaction. There are a set a rules that we use to determine oxidation number. Group 1A elements (alkalai metals) always have an oxidation of +1. Group 2A elements (alkaline earth metals) always have an oxidation number of +2. Elements on their own have an oxidation number of 0, and monatomic ions have an oxidation number that is equal to the ionic charge.
published: 15 Jun 2015 -

Practice determining oxidation states | Chemistry | Khan Academy
"Determining oxidation numbers in magnesium oxide and magnesium hydroxide. Watch the next lesson: https://www.khanacademy.org/science/chemistry/oxidation-reduction/redox-oxidation-reduction/v/unusual-oxygen-oxidation-states?utm_source=YT&utm;_medium=Desc&utm;_campaign=chemistry Missed the previous lesson? https://www.khanacademy.org/science/chemistry/oxidation-reduction/redox-oxidation-reduction/v/oxidation-state-trends-in-periodic-table?utm_source=YT&utm;_medium=Desc&utm;_campaign=chemistry Chemistry on Khan Academy: Did you know that everything is made out of chemicals? Chemistry is the study of matter: its composition, properties, and reactivity. This material roughly covers a first-year high school or college course, and a good understanding of algebra is helpful. About Khan Academy: ...
published: 09 Jan 2014 -

How to Figure out Oxidation Numbers
How to assign oxidation numbers to the atoms in a molecule. 1. Elements have oxidation number = 0 2. Hydrogen's always +1 (except in "hydrides") 3. Oxygen's always -2 (except in "peroxides") 4. Other atoms get the charge they prefer, as long as the sum of oxidation numbers for all atoms = the total charge on the atom.
published: 27 Mar 2012 -

-

4.1 Introduction to Oxidation state
In this video I have taught what is oxidation state and what the most easy method to find the oxidation state. As knowledge of oxidation state is needed throughout the chemistry,this is a must watch video. Enjoy it.
published: 28 Mar 2014 -

How to Calculate Oxidation Number Practice Problems
Many practice problems for how to calculate and determine oxidation numbers, often referred to as oxidation states. To figure out oxidation numbers for elements in a compound, we have to look on the periodic table and consult a list of rules. For neutral compounds, the oxidation numbers add up to zero. For polyatomic ions, the oxidation numbers add up to charge of the ion.
published: 17 Jun 2015 -

Redox Reactions: Crash Course Chemistry #10
All the magic that we know is in the transfer of electrons. Reduction (gaining electrons) and oxidation (the loss of electrons) combine to form Redox chemistry, which contains the majority of chemical reactions. As electrons jump from atom to atom, they carry energy with them, and that transfer of energy is what makes all life on earth possible. **Special Thanks to Matt Young at the University of Montana (Geosciences Department, Environmental Biogeochemistry Lab) who helped with the chemical demonstrations.** Oxidation 1:42 Reduction 1:03 Oxidation Numbers 3:29 Redox Reactions 5:59 Oxidation Reactions 6:28 Balancing Oxidation Reactions 7:18 Also thank you to the following chemistry teachers for assistance: James Sarbinoff Rachel Wentz Edi González Lucas Moore Chris Conley Addie Clark J...
published: 23 Apr 2013 -

Assigning Oxidation Numbers - Chemistry Tutorial
This chemistry tutorial discusses how to assign oxidation numbers and includes examples of how to determine the oxidation numbers in a compound following some simple rules. https://www.thechemistrysolution.com
published: 27 Nov 2011 -

Oxidation Numbers & States Explained - Rules, Polyatomic Ions, Compounds, & Transition Metals
This chemistry video tutorial shows you how to determine the oxidation state or oxidation number of an element in a compound or a transition metal within a polyatomic ion. This video contains plenty of examples and practice problems for you to work on. Here is a list of topics: 1. Assigning Oxidation Numbers To Elements in Compounds 2. Determining The Oxidation States of Transition Metals 3. Calculating The Oxidation Number of a Element in a polyatomic ion 4. Oxidation Number Rules - Pure Elements Always Zero 5. Oxidation States - Electronegativity - Fluorine is -1 6. Oxidation Rules - Oxygen Usually is -2 in Oxide and -2 in Peroxide 7. Hydrogen is +1 when bonded to a nonmetal and -1 when bonded to a metal 8. Transition Metals - Multiple Oxidation States
published: 05 Oct 2015 -

Oxidation state trends in periodic table | Chemistry | Khan Academy
Trends in common oxidation states for main group elements. Watch the next lesson: https://www.khanacademy.org/science/chemistry/oxidation-reduction/redox-oxidation-reduction/v/practice-determining-oxidation-states?utm_source=YT&utm;_medium=Desc&utm;_campaign=chemistry Missed the previous lesson? https://www.khanacademy.org/science/chemistry/oxidation-reduction/redox-oxidation-reduction/v/introduction-to-oxidation-and-reduction?utm_source=YT&utm;_medium=Desc&utm;_campaign=chemistry Chemistry on Khan Academy: Did you know that everything is made out of chemicals? Chemistry is the study of matter: its composition, properties, and reactivity. This material roughly covers a first-year high school or college course, and a good understanding of algebra is helpful. About Khan Academy: Khan Academ...
published: 08 Jan 2014
How to Calculate Oxidation Numbers Introduction
- Order: Reorder
- Duration: 13:26
- Updated: 15 Jun 2015
- views: 262444
- published: 15 Jun 2015
- views: 262444
Practice determining oxidation states | Chemistry | Khan Academy
- Order: Reorder
- Duration: 4:27
- Updated: 09 Jan 2014
- views: 178529
- published: 09 Jan 2014
- views: 178529
How to Figure out Oxidation Numbers
- Order: Reorder
- Duration: 9:37
- Updated: 27 Mar 2012
- views: 543625
- published: 27 Mar 2012
- views: 543625
Introduction to Oxidation States
- Order: Reorder
- Duration: 17:39
- Updated: 10 Sep 2009
- views: 366668
4.1 Introduction to Oxidation state
- Order: Reorder
- Duration: 30:38
- Updated: 28 Mar 2014
- views: 36590
- published: 28 Mar 2014
- views: 36590
How to Calculate Oxidation Number Practice Problems
- Order: Reorder
- Duration: 15:25
- Updated: 17 Jun 2015
- views: 111717
- published: 17 Jun 2015
- views: 111717
Redox Reactions: Crash Course Chemistry #10
- Order: Reorder
- Duration: 11:13
- Updated: 23 Apr 2013
- views: 1161912
- published: 23 Apr 2013
- views: 1161912
Assigning Oxidation Numbers - Chemistry Tutorial
- Order: Reorder
- Duration: 6:00
- Updated: 27 Nov 2011
- views: 117447
- published: 27 Nov 2011
- views: 117447
Oxidation Numbers & States Explained - Rules, Polyatomic Ions, Compounds, & Transition Metals
- Order: Reorder
- Duration: 19:46
- Updated: 05 Oct 2015
- views: 5590
- published: 05 Oct 2015
- views: 5590
Oxidation state trends in periodic table | Chemistry | Khan Academy
- Order: Reorder
- Duration: 8:38
- Updated: 08 Jan 2014
- views: 139815
- published: 08 Jan 2014
- views: 139815
-

30 Oxidation States and Mechanisms
published: 02 Nov 2016 -

4.1 Introduction to Oxidation state
In this video I have taught what is oxidation state and what the most easy method to find the oxidation state. As knowledge of oxidation state is needed throughout the chemistry,this is a must watch video. Enjoy it.
published: 28 Mar 2014 -

Variable Oxidation States of Transition Metals
published: 27 May 2013 -

-

Oxidation State: Redox Reaction 02/14
published: 27 Feb 2016 -

Oxidation State of halogens: Redox Reaction 03/14
published: 27 Feb 2016 -

Introduction to Oxidations states of organic compounds.mp4
published: 08 Jun 2012 -

Oxidation state of special elements: Redox Reaction 05/14
published: 27 Feb 2016 -

Oxidation State of Sulphur and exceptional oxygen: Redox Reaction 04/14
published: 27 Feb 2016 -

Chemical Bonding # Oxidations states of organic compounds
published: 05 Apr 2013
30 Oxidation States and Mechanisms
- Order: Reorder
- Duration: 46:58
- Updated: 02 Nov 2016
- views: 0
- published: 02 Nov 2016
- views: 0
4.1 Introduction to Oxidation state
- Order: Reorder
- Duration: 30:38
- Updated: 28 Mar 2014
- views: 36590
- published: 28 Mar 2014
- views: 36590
Variable Oxidation States of Transition Metals
- Order: Reorder
- Duration: 32:35
- Updated: 27 May 2013
- views: 6836
- published: 27 May 2013
- views: 6836
Oxidation states, Atomic & Ionic sizes
- Order: Reorder
- Duration: 36:03
- Updated: 04 Dec 2013
- views: 514
Oxidation State: Redox Reaction 02/14
- Order: Reorder
- Duration: 43:24
- Updated: 27 Feb 2016
- views: 9
- published: 27 Feb 2016
- views: 9
Oxidation State of halogens: Redox Reaction 03/14
- Order: Reorder
- Duration: 33:00
- Updated: 27 Feb 2016
- views: 12
- published: 27 Feb 2016
- views: 12
Introduction to Oxidations states of organic compounds.mp4
- Order: Reorder
- Duration: 33:34
- Updated: 08 Jun 2012
- views: 389
- published: 08 Jun 2012
- views: 389
Oxidation state of special elements: Redox Reaction 05/14
- Order: Reorder
- Duration: 38:20
- Updated: 27 Feb 2016
- views: 2
- published: 27 Feb 2016
- views: 2
Oxidation State of Sulphur and exceptional oxygen: Redox Reaction 04/14
- Order: Reorder
- Duration: 40:16
- Updated: 27 Feb 2016
- views: 11
- published: 27 Feb 2016
- views: 11
Chemical Bonding # Oxidations states of organic compounds
- Order: Reorder
- Duration: 33:34
- Updated: 05 Apr 2013
- views: 8
- published: 05 Apr 2013
- views: 8


- Playlist
- Chat

How to Calculate Oxidation Numbers Introduction
- Report rights infringement
- published: 15 Jun 2015
- views: 262444

Practice determining oxidation states | Chemistry | Khan Academy
- Report rights infringement
- published: 09 Jan 2014
- views: 178529

How to Figure out Oxidation Numbers
- Report rights infringement
- published: 27 Mar 2012
- views: 543625

Introduction to Oxidation States
- Report rights infringement
- published: 10 Sep 2009
- views: 366668

4.1 Introduction to Oxidation state
- Report rights infringement
- published: 28 Mar 2014
- views: 36590

How to Calculate Oxidation Number Practice Problems
- Report rights infringement
- published: 17 Jun 2015
- views: 111717

Redox Reactions: Crash Course Chemistry #10
- Report rights infringement
- published: 23 Apr 2013
- views: 1161912

Assigning Oxidation Numbers - Chemistry Tutorial
- Report rights infringement
- published: 27 Nov 2011
- views: 117447

Oxidation Numbers & States Explained - Rules, Polyatomic Ions, Compounds, & Transition Metals
- Report rights infringement
- published: 05 Oct 2015
- views: 5590

Oxidation state trends in periodic table | Chemistry | Khan Academy
- Report rights infringement
- published: 08 Jan 2014
- views: 139815

- Playlist
- Chat

How to Calculate Oxidation Numbers Introduction
- Report rights infringement
- published: 15 Jun 2015
- views: 262444

Practice determining oxidation states | Chemistry | Khan Academy
- Report rights infringement
- published: 09 Jan 2014
- views: 178529

How to Figure out Oxidation Numbers
- Report rights infringement
- published: 27 Mar 2012
- views: 543625

Introduction to Oxidation States
- Report rights infringement
- published: 10 Sep 2009
- views: 366668

4.1 Introduction to Oxidation state
- Report rights infringement
- published: 28 Mar 2014
- views: 36590

How to Calculate Oxidation Number Practice Problems
- Report rights infringement
- published: 17 Jun 2015
- views: 111717

Redox Reactions: Crash Course Chemistry #10
- Report rights infringement
- published: 23 Apr 2013
- views: 1161912

Assigning Oxidation Numbers - Chemistry Tutorial
- Report rights infringement
- published: 27 Nov 2011
- views: 117447

Oxidation Numbers & States Explained - Rules, Polyatomic Ions, Compounds, & Transition Metals
- Report rights infringement
- published: 05 Oct 2015
- views: 5590

Oxidation state trends in periodic table | Chemistry | Khan Academy
- Report rights infringement
- published: 08 Jan 2014
- views: 139815

- Playlist
- Chat

30 Oxidation States and Mechanisms
- Report rights infringement
- published: 02 Nov 2016
- views: 0

4.1 Introduction to Oxidation state
- Report rights infringement
- published: 28 Mar 2014
- views: 36590

Variable Oxidation States of Transition Metals
- Report rights infringement
- published: 27 May 2013
- views: 6836

Oxidation states, Atomic & Ionic sizes
- Report rights infringement
- published: 04 Dec 2013
- views: 514

Oxidation State: Redox Reaction 02/14
- Report rights infringement
- published: 27 Feb 2016
- views: 9

Oxidation State of halogens: Redox Reaction 03/14
- Report rights infringement
- published: 27 Feb 2016
- views: 12

Introduction to Oxidations states of organic compounds.mp4
- Report rights infringement
- published: 08 Jun 2012
- views: 389

Oxidation state of special elements: Redox Reaction 05/14
- Report rights infringement
- published: 27 Feb 2016
- views: 2

Oxidation State of Sulphur and exceptional oxygen: Redox Reaction 04/14
- Report rights infringement
- published: 27 Feb 2016
- views: 11

Chemical Bonding # Oxidations states of organic compounds
- Report rights infringement
- published: 05 Apr 2013
- views: 8
Bilderberg 2017: secret meeting of global leaders could prove a problem for Trump
Edit The Guardian 01 Jun 2017Giant Crack in Antarctica’s Larsen C Ice Shelf Grew 11 Miles in Just 6 Days
Edit Newsweek 01 Jun 2017UKIP's Nigel Farage Called 'Person Of Interest' In FBI Collusion Investigation: Report
Edit WorldNews.com 01 Jun 2017Comedian Kathy Griffin Loses CNN Job In Fallout To Controversial Trump Photo
Edit WorldNews.com 31 May 2017Susan Rice Took No Inappropriate Action In Surveillance Unmasking, Bi-Partisan Lawmakers Say
Edit WorldNews.com 01 Jun 2017Nano Metal Oxide Market is expected to reach USD 9,48 billion by 2025
Edit Market Watch 01 Jun 2017Global Magnesium Oxide Boards Market 2017 - Industry Growth, Analysis, Size and Share to 2022
Edit Community news 01 Jun 2017Global Oxidizing Catalytic Converters Market Research Report 2017
Edit Community news 01 Jun 2017North America Magnesium Oxide Boards Market 2017 : Technological advancements, Financial Plan 2017 to 2022
Edit Community news 01 Jun 2017MacDonald Mines Exploration Ltd.: Airborne Magnetics Corroborates Interpreted >2km Strike Length of Oxide Sands at its Wawa-Holdsworth Project
Edit Market Watch 01 Jun 2017Medallion and Rare Earth Salts Agree to Produce Rare Earth Products
Edit Market Watch 01 Jun 2017Market Professional Report-United States Transparent Conducting Oxide (TCO) Glass Market Report 2017
Edit Community news 01 Jun 2017Dental Wound Dressings Market Research Report: Forecast up to 2024
Edit Community news 01 Jun 2017Audi used software to cheat diesel emissions tests, says German minister
Edit Irish Independent 01 Jun 2017Man pleads guilty to scamming $4M-plus from the government
Edit The Miami Herald 01 Jun 20177 dry fruits you should include in your diet to stay healthy
Edit Hindustan Times 01 Jun 2017Orca Gold Reports Voting Results from Annual Meeting and Provides Highlights of Presentation Given on Revised PEA
Edit Market Watch 01 Jun 2017- 1
- 2
- 3
- 4
- 5
- Next page »




























