- published: 16 Dec 2015
- views: 19884
-
remove the playlistThermodynamics
- remove the playlistThermodynamics
- published: 03 Dec 2012
- views: 81417
- published: 16 Sep 2009
- views: 760014
- published: 25 Apr 2008
- views: 806492
- published: 23 Sep 2008
- views: 288151
- published: 30 Jul 2015
- views: 22869
- published: 17 Jul 2013
- views: 80526

Thermodynamics
Thermodynamics is a branch of physics concerned with heat and temperature and their relation to energy and work. It defines macroscopic variables, such as internal energy, entropy, and pressure, that partly describe a body of matter or radiation. It states that the behavior of those variables is subject to general constraints, that are common to all materials, beyond the peculiar properties of particular materials. These general constraints are expressed in the four laws of thermodynamics. Thermodynamics describes the bulk behavior of the body, not the microscopic behaviors of the very large numbers of its microscopic constituents, such as molecules. The basic results of thermodynamics rely on the existence of idealized states of thermodynamic equilibrium. Its laws are explained by statistical mechanics, in terms of the microscopic constituents.
Thermodynamics applies to a wide variety of topics in science and engineering, especially physical chemistry, chemical engineering and mechanical engineering.
This article is licensed under the Creative Commons Attribution-ShareAlike 3.0 Unported License, which means that you can copy and modify it as long as the entire work (including additions) remains under this license.

Michio Kaku
Michio Kaku (/ˈmiːtʃioʊ ˈkɑːkuː/; born January 24, 1947) is a Japanese-American futurist, theoretical physicist and popularizer of science. Kaku is a Professor of Theoretical Physics at the City College of New York. He has written several books about physics and related topics, has made frequent appearances on radio, television, and film, and writes online blogs and articles. He has written three New York Times Best Sellers: Physics of the Impossible (2008), Physics of the Future (2011), and The Future of the Mind (2014). Kaku has hosted several TV specials for the BBC, the Discovery Channel, the History Channel, and the Science Channel.
Early life and education
Kaku was born in San Jose, California, to Japanese American parents. His father, born in California and educated in both Japan and the United States, was fluent in Japanese and English. Both his parents were interned in the Tule Lake War Relocation Center during World War II, where they met and where his older brother was born.
This article is licensed under the Creative Commons Attribution-ShareAlike 3.0 Unported License, which means that you can copy and modify it as long as the entire work (including additions) remains under this license.
- Loading...

-
 8:12
8:12The Laws of Thermodynamics, Entropy, and Gibbs Free Energy
The Laws of Thermodynamics, Entropy, and Gibbs Free EnergyThe Laws of Thermodynamics, Entropy, and Gibbs Free Energy
We've all heard of the Laws of Thermodynamics, but what are they really? What the heck is entropy and what does it mean for the fate of the universe? How does soap work?! So many questions answered in this clip! Enjoy! Subscribe: http://bit.ly/ProfDaveSubscribe ProfessorDaveExplains@gmail.com http://professordaveexplains.com http://facebook.com/ProfessorDaveExpl... http://twitter.com/DaveExplains General Chemistry - Online Tutorials: http://bit.ly/ProfDaveGenChem Organic Chemistry - Online Tutorials: http://bit.ly/ProfDaveOrgChem Science for Common Folk - Online Tutorials: http://bit.ly/ProfDaveScience4CommonFolk -
 46:46
46:46Lec 1 | MIT 5.60 Thermodynamics & Kinetics, Spring 2008
Lec 1 | MIT 5.60 Thermodynamics & Kinetics, Spring 2008 -
 10:14
10:14The 0th and 1st Laws of Thermodynamics | Doc Physics
The 0th and 1st Laws of Thermodynamics | Doc PhysicsThe 0th and 1st Laws of Thermodynamics | Doc Physics
These are pretty easy stuff, but they make a nice foundation for what's to come. -
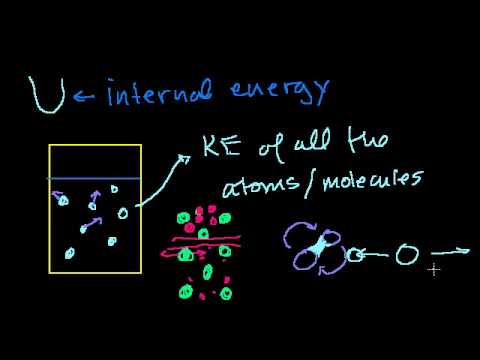 17:40
17:40First law of thermodynamics / internal energy | Thermodynamics | Physics | Khan Academy
First law of thermodynamics / internal energy | Thermodynamics | Physics | Khan AcademyFirst law of thermodynamics / internal energy | Thermodynamics | Physics | Khan Academy
First law of thermodynamic and internal energy. Created by Sal Khan. Watch the next lesson: https://www.khanacademy.org/science/physics/thermodynamics/laws-of-thermodynamics/v/more-on-internal-energy?utm_source=YT&utm;_medium=Desc&utm;_campaign=physics Missed the previous lesson? https://www.khanacademy.org/science/physics/thermodynamics/laws-of-thermodynamics/v/quasistatic-and-reversible-processes?utm_source=YT&utm;_medium=Desc&utm;_campaign=physics Physics on Khan Academy: Physics is the study of the basic principles that govern the physical world around us. We'll start by looking at motion itself. Then, we'll learn about forces, momentum, energy, and other concepts in lots of different physical situations. To get the most out of physics, you'll need a solid understanding of algebra and a basic understanding of trigonometry. About Khan Academy: Khan Academy offers practice exercises, instructional videos, and a personalized learning dashboard that empower learners to study at their own pace in and outside of the classroom. We tackle math, science, computer programming, history, art history, economics, and more. Our math missions guide learners from kindergarten to calculus using state-of-the-art, adaptive technology that identifies strengths and learning gaps. We've also partnered with institutions like NASA, The Museum of Modern Art, The California Academy of Sciences, and MIT to offer specialized content. For free. For everyone. Forever. #YouCanLearnAnything Subscribe to Khan Academy’s Physics channel: https://www.youtube.com/channel/UC0oGarQW2lE5PxhGoQAKV7Q?sub_confirmation=1 Subscribe to Khan Academy: https://www.youtube.com/subscription_center?add_user=khanacademy -
 9:50
9:50Thermodynamics part 1: Molecular theory of gases | Physics | Khan Academy
Thermodynamics part 1: Molecular theory of gases | Physics | Khan AcademyThermodynamics part 1: Molecular theory of gases | Physics | Khan Academy
Intuition of how gases generate pressure in a container and why pressure x volume is proportional to the combined kinetic energy of the molecules in the volume. Created by Sal Khan. Watch the next lesson: https://www.khanacademy.org/science/physics/thermodynamics/temp-kinetic-theory-ideal-gas-law/v/thermodynamics-part-2?utm_source=YT&utm;_medium=Desc&utm;_campaign=physics Missed the previous lesson? https://www.khanacademy.org/science/physics/fluids/fluid-dynamics/v/surface-tension-and-adhesion?utm_source=YT&utm;_medium=Desc&utm;_campaign=physics Physics on Khan Academy: Physics is the study of the basic principles that govern the physical world around us. We'll start by looking at motion itself. Then, we'll learn about forces, momentum, energy, and other concepts in lots of different physical situations. To get the most out of physics, you'll need a solid understanding of algebra and a basic understanding of trigonometry. About Khan Academy: Khan Academy offers practice exercises, instructional videos, and a personalized learning dashboard that empower learners to study at their own pace in and outside of the classroom. We tackle math, science, computer programming, history, art history, economics, and more. Our math missions guide learners from kindergarten to calculus using state-of-the-art, adaptive technology that identifies strengths and learning gaps. We've also partnered with institutions like NASA, The Museum of Modern Art, The California Academy of Sciences, and MIT to offer specialized content. For free. For everyone. Forever. #YouCanLearnAnything Subscribe to Khan Academy’s Physics channel: https://www.youtube.com/channel/UC0oGarQW2lE5PxhGoQAKV7Q?sub_confirmation=1 Subscribe to Khan Academy: https://www.youtube.com/subscription_center?add_user=khanacademy -
 35:56
35:56Thermodynamics and the End of the Universe: Energy, Entropy, and the fundamental laws of physics.
Thermodynamics and the End of the Universe: Energy, Entropy, and the fundamental laws of physics. -
 71:28
71:2821. Thermodynamics
21. Thermodynamics21. Thermodynamics
For more information about Professor Shankar's book based on the lectures from this course, Fundamentals of Physics: Mechanics, Relativity, and Thermodynamics, visit http://bit.ly/1jFIqNu. Fundamentals of Physics (PHYS 200) This is the first of a series of lectures on thermodynamics. The discussion begins with understanding "temperature." Zeroth's law is introduced and explained. Concepts such as "absolute zero" and "triple point of water" are defined. Measuring temperature through a number of instruments is addressed as well as the different scales of measurement. The second half of the lecture is devoted to heat and heat transfer. Concepts such as "convection" and "conduction" are explained thoroughly. 00:00 - Chapter 1. Temperature as a Macroscopic Thermodynamic Property 06:45 - Chapter 2. Calibrating Temperature Instruments 22:25 - Chapter 3. Absolute Zero, Triple Point of Water, The Kelvin 28:55 - Chapter 4. Specific Heat and Other Thermal Properties of Materials 43:17 - Chapter 5. Phase Change 55:06 - Chapter 6. Heat Transfer by Radiation, Convection and Conduction 01:03:27 - Chapter 7. Heat as Atomic Kinetic Energy and its Measurement Complete course materials are available at the Yale Online website: online.yale.edu This course was recorded in Fall 2006. -
 6:46
6:46Michio Kaku (2014) on "The Laws of Thermodynamics and Fate of The Universe"
Michio Kaku (2014) on "The Laws of Thermodynamics and Fate of The Universe"Michio Kaku (2014) on "The Laws of Thermodynamics and Fate of The Universe"
Subscribe For Michio Kaku's Speeches, Interviews, and Debates! -
 4:47
4:47Second Law of Thermodynamics
Second Law of ThermodynamicsSecond Law of Thermodynamics
133 - Second Law of Thermodynamics In this video Paul Andersen explains how the second law of thermodynamics applies to reversible and irreversible processes. In a reversible process the net change in entropy is zero. In and irreversible process the entropy will always increase in a closed system. The entropy measures the disorder in the entire system and will move in the direction of time’s arrow. Several example videos of increasing entropy are included. Do you speak another language? Help me translate my videos: http://www.bozemanscience.com/translations/ Music Attribution Title: String Theory Artist: Herman Jolly http://sunsetvalley.bandcamp.com/track/string-theory All of the images are licensed under creative commons and public domain licensing: Ebeling, ESA/Hubble, NASA and H. English: At First Glance, the Scatter of Pale Dots on This NASA/ESA Hubble Space Telescope Image Looks like a Snowstorm in the Night Sky. But Almost Every One of These Delicate Snowflakes Is a Distant Galaxy in the Cluster MACS J0717.5+3745 and Each Is Home to Billions of Stars., August 16, 2010. http://spacetelescope.org/images/potw1017a/. http://commons.wikimedia.org/wiki/File:Cluster_MACS_J0717.5%2B3745.jpg. -
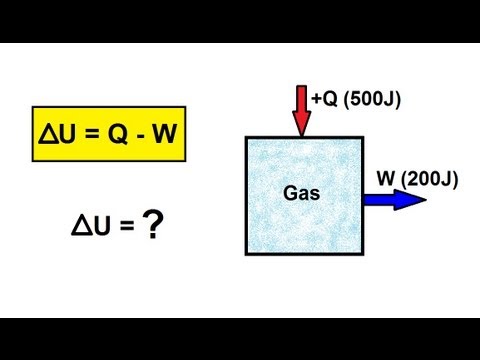 3:21
3:21Physics - Thermodynamics: (1 of 22) What is the First Law of Thermodynamics?
Physics - Thermodynamics: (1 of 22) What is the First Law of Thermodynamics?Physics - Thermodynamics: (1 of 22) What is the First Law of Thermodynamics?
Visit http://ilectureonline.com for more math and science lectures! In this video I will explain and give an example of the First Law of Thermodynamics.
-

The Laws of Thermodynamics, Entropy, and Gibbs Free Energy
We've all heard of the Laws of Thermodynamics, but what are they really? What the heck is entropy and what does it mean for the fate of the universe? How does soap work?! So many questions answered in this clip! Enjoy! Subscribe: http://bit.ly/ProfDaveSubscribe ProfessorDaveExplains@gmail.com http://professordaveexplains.com http://facebook.com/ProfessorDaveExpl... http://twitter.com/DaveExplains General Chemistry - Online Tutorials: http://bit.ly/ProfDaveGenChem Organic Chemistry - Online Tutorials: http://bit.ly/ProfDaveOrgChem Science for Common Folk - Online Tutorials: http://bit.ly/ProfDaveScience4CommonFolk
published: 16 Dec 2015 -

Lec 1 | MIT 5.60 Thermodynamics & Kinetics, Spring 2008
Lecture 1: State of a system, 0th law, equation of state. View the complete course at: http://ocw.mit.edu/5-60S08 License: Creative Commons BY-NC-SA More information at http://ocw.mit.edu/terms More courses at http://ocw.mit.edu
published: 12 Dec 2008 -

The 0th and 1st Laws of Thermodynamics | Doc Physics
These are pretty easy stuff, but they make a nice foundation for what's to come.
published: 03 Dec 2012 -

First law of thermodynamics / internal energy | Thermodynamics | Physics | Khan Academy
First law of thermodynamic and internal energy. Created by Sal Khan. Watch the next lesson: https://www.khanacademy.org/science/physics/thermodynamics/laws-of-thermodynamics/v/more-on-internal-energy?utm_source=YT&utm;_medium=Desc&utm;_campaign=physics Missed the previous lesson? https://www.khanacademy.org/science/physics/thermodynamics/laws-of-thermodynamics/v/quasistatic-and-reversible-processes?utm_source=YT&utm;_medium=Desc&utm;_campaign=physics Physics on Khan Academy: Physics is the study of the basic principles that govern the physical world around us. We'll start by looking at motion itself. Then, we'll learn about forces, momentum, energy, and other concepts in lots of different physical situations. To get the most out of physics, you'll need a solid understanding of algebra and a...
published: 16 Sep 2009 -

Thermodynamics part 1: Molecular theory of gases | Physics | Khan Academy
Intuition of how gases generate pressure in a container and why pressure x volume is proportional to the combined kinetic energy of the molecules in the volume. Created by Sal Khan. Watch the next lesson: https://www.khanacademy.org/science/physics/thermodynamics/temp-kinetic-theory-ideal-gas-law/v/thermodynamics-part-2?utm_source=YT&utm;_medium=Desc&utm;_campaign=physics Missed the previous lesson? https://www.khanacademy.org/science/physics/fluids/fluid-dynamics/v/surface-tension-and-adhesion?utm_source=YT&utm;_medium=Desc&utm;_campaign=physics Physics on Khan Academy: Physics is the study of the basic principles that govern the physical world around us. We'll start by looking at motion itself. Then, we'll learn about forces, momentum, energy, and other concepts in lots of different physi...
published: 25 Apr 2008 -

-

21. Thermodynamics
For more information about Professor Shankar's book based on the lectures from this course, Fundamentals of Physics: Mechanics, Relativity, and Thermodynamics, visit http://bit.ly/1jFIqNu. Fundamentals of Physics (PHYS 200) This is the first of a series of lectures on thermodynamics. The discussion begins with understanding "temperature." Zeroth's law is introduced and explained. Concepts such as "absolute zero" and "triple point of water" are defined. Measuring temperature through a number of instruments is addressed as well as the different scales of measurement. The second half of the lecture is devoted to heat and heat transfer. Concepts such as "convection" and "conduction" are explained thoroughly. 00:00 - Chapter 1. Temperature as a Macroscopic Thermodynamic Property 06:45 - Chap...
published: 23 Sep 2008 -

Michio Kaku (2014) on "The Laws of Thermodynamics and Fate of The Universe"
Subscribe For Michio Kaku's Speeches, Interviews, and Debates!
published: 04 Jul 2014 -

Second Law of Thermodynamics
133 - Second Law of Thermodynamics In this video Paul Andersen explains how the second law of thermodynamics applies to reversible and irreversible processes. In a reversible process the net change in entropy is zero. In and irreversible process the entropy will always increase in a closed system. The entropy measures the disorder in the entire system and will move in the direction of time’s arrow. Several example videos of increasing entropy are included. Do you speak another language? Help me translate my videos: http://www.bozemanscience.com/translations/ Music Attribution Title: String Theory Artist: Herman Jolly http://sunsetvalley.bandcamp.com/track/string-theory All of the images are licensed under creative commons and public domain licensing: Ebeling, ESA/Hubble, NASA and...
published: 30 Jul 2015 -

Physics - Thermodynamics: (1 of 22) What is the First Law of Thermodynamics?
Visit http://ilectureonline.com for more math and science lectures! In this video I will explain and give an example of the First Law of Thermodynamics.
published: 17 Jul 2013
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy
- Order: Reorder
- Duration: 8:12
- Updated: 16 Dec 2015
- views: 19884
- published: 16 Dec 2015
- views: 19884
Lec 1 | MIT 5.60 Thermodynamics & Kinetics, Spring 2008
- Order: Reorder
- Duration: 46:46
- Updated: 12 Dec 2008
- views: 634584
The 0th and 1st Laws of Thermodynamics | Doc Physics
- Order: Reorder
- Duration: 10:14
- Updated: 03 Dec 2012
- views: 81417
- published: 03 Dec 2012
- views: 81417
First law of thermodynamics / internal energy | Thermodynamics | Physics | Khan Academy
- Order: Reorder
- Duration: 17:40
- Updated: 16 Sep 2009
- views: 760014
- published: 16 Sep 2009
- views: 760014
Thermodynamics part 1: Molecular theory of gases | Physics | Khan Academy
- Order: Reorder
- Duration: 9:50
- Updated: 25 Apr 2008
- views: 806492
- published: 25 Apr 2008
- views: 806492
Thermodynamics and the End of the Universe: Energy, Entropy, and the fundamental laws of physics.
- Order: Reorder
- Duration: 35:56
- Updated: 08 Sep 2013
- views: 227919
21. Thermodynamics
- Order: Reorder
- Duration: 71:28
- Updated: 23 Sep 2008
- views: 288151
- published: 23 Sep 2008
- views: 288151
Michio Kaku (2014) on "The Laws of Thermodynamics and Fate of The Universe"
- Order: Reorder
- Duration: 6:46
- Updated: 04 Jul 2014
- views: 30924
Second Law of Thermodynamics
- Order: Reorder
- Duration: 4:47
- Updated: 30 Jul 2015
- views: 22869
- published: 30 Jul 2015
- views: 22869
Physics - Thermodynamics: (1 of 22) What is the First Law of Thermodynamics?
- Order: Reorder
- Duration: 3:21
- Updated: 17 Jul 2013
- views: 80526
- published: 17 Jul 2013
- views: 80526
- Playlist
- Chat
- Playlist
- Chat

The Laws of Thermodynamics, Entropy, and Gibbs Free Energy
- Report rights infringement
- published: 16 Dec 2015
- views: 19884

Lec 1 | MIT 5.60 Thermodynamics & Kinetics, Spring 2008
- Report rights infringement
- published: 12 Dec 2008
- views: 634584

The 0th and 1st Laws of Thermodynamics | Doc Physics
- Report rights infringement
- published: 03 Dec 2012
- views: 81417

First law of thermodynamics / internal energy | Thermodynamics | Physics | Khan Academy
- Report rights infringement
- published: 16 Sep 2009
- views: 760014

Thermodynamics part 1: Molecular theory of gases | Physics | Khan Academy
- Report rights infringement
- published: 25 Apr 2008
- views: 806492

Thermodynamics and the End of the Universe: Energy, Entropy, and the fundamental laws of physics.
- Report rights infringement
- published: 08 Sep 2013
- views: 227919

21. Thermodynamics
- Report rights infringement
- published: 23 Sep 2008
- views: 288151

Michio Kaku (2014) on "The Laws of Thermodynamics and Fate of The Universe"
- Report rights infringement
- published: 04 Jul 2014
- views: 30924

Second Law of Thermodynamics
- Report rights infringement
- published: 30 Jul 2015
- views: 22869

Physics - Thermodynamics: (1 of 22) What is the First Law of Thermodynamics?
- Report rights infringement
- published: 17 Jul 2013
- views: 80526
[VIDEO]: Assad Blames US For Airstrikes On Syrian Troops
Edit WorldNews.com 22 Sep 2016The Climate Change Debate is Over
Edit CounterPunch 22 Sep 2016Aboriginal Australians are Earth's oldest civilization: DNA study
Edit CNN 22 Sep 2016[VIDEO]: Alien Fleet Passes Through Our Solar System And Ignores Us
Edit WorldNews.com 21 Sep 2016Artificial intelligence helps in the discovery of new materials
Edit Science Daily 22 Sep 2016Reservoir engineering software workflow: a new partnership between IFPEN, Beicip-Franlab and KAPPA (IFP Energies nouvelles)
Edit Public Technologies 22 Sep 2016New Intuitive Software For Reichert4spr 4-Channel Surface Plasmon Resonance Instrument (Ametek Inc)
Edit Public Technologies 22 Sep 2016Faculty Books and Articles Published (California State University, Fullerton)
Edit Public Technologies 21 Sep 2016Science on Tap talk to feature the development of sustainable electronics (Purdue University)
Edit Public Technologies 21 Sep 2016Not All Who Wear Fitness Trackers Are Lost
Edit The Atlantic 21 Sep 2016Mechanical approach to chemical transport [Chemistry]>
Edit PNAS 20 Sep 2016Theory of {delta}-plutonium's temperature response [Physics]>
Edit PNAS 20 Sep 2016Artificial Intelligence Helps in the Discovery of New Materials (Universität Basel)
Edit Public Technologies 20 Sep 2016Aviadvigatel JSC are among the winners of contest «Young Analyst» (OAO Permskiye motory)
Edit Public Technologies 20 Sep 2016In exploring the ‘now,’ new book links flow of time with Big Bang (University of California - Berkeley)
Edit Public Technologies 20 Sep 2016Coed's sliming pinned on display pod on the lam
Edit Arkansas Online 17 Sep 2016Meet our new engineering faculty (Saint Ambrose University)
Edit Public Technologies 16 Sep 2016- 1
- 2
- 3
- 4
- 5
- Next page »








