- published: 28 Nov 2012
- views: 146168
-
remove the playlistJ. J. Thomson
-
remove the playlistLatest Videos
-
remove the playlistLongest Videos
- remove the playlistJ. J. Thomson
- remove the playlistLatest Videos
- remove the playlistLongest Videos
- published: 14 May 2015
- views: 8034
- published: 02 Feb 2011
- views: 137049
- published: 26 Nov 2010
- views: 40489
- published: 01 Mar 2008
- views: 322569
- published: 29 Sep 2013
- views: 26862
- published: 01 Oct 2008
- views: 226127
- published: 07 Apr 2012
- views: 169212

Sir Joseph John "J. J." Thomson, OM, FRS (18 December 1856 – 30 August 1940) was a British physicist and Nobel laureate. He is credited with discovering electrons and isotopes, and inventing the mass spectrometer. Thomson was awarded the 1906 Nobel Prize in Physics for the discovery of the electron and for his work on the conduction of electricity in gases.
Joseph John Thomson was born in 1856 in Cheetham Hill, Manchester, England. His mother, Emma Swindells, came from a local textile family. His father, Joseph James Thomson, ran an antiquarian bookshop founded by a great-grandfather from Scotland (hence the Scottish spelling of his surname). He had a brother two years younger than he, Frederick Vernon Thomson.
His early education took place in small private schools where he demonstrated great talent and interest in science. In 1870 he was admitted to Owens College. Being only 14 years old at the time, he was unusually young. His parents planned to enroll him as an apprentice engineer to Sharp-Stewart & Co., a locomotive manufacturer, but these plans were cut short when his father died in 1873. He moved on to Trinity College, Cambridge in 1876. In 1880, he obtained his BA in mathematics (Second Wrangler and 2nd Smith's prize) and MA (with Adams Prize) in 1883. In 1884 he became Cavendish Professor of Physics. One of his students was Ernest Rutherford, who would later succeed him in the post. In 1890 he married Rose Elisabeth Paget, daughter of Sir George Edward Paget, KCB, a physician and then Regius Professor of Physic at Cambridge. He had one son, George Paget Thomson, and one daughter, Joan Paget Thomson, with her. One of Thomson's greatest contributions to modern science was in his role as a highly gifted teacher, as seven of his research assistants and his aforementioned son won Nobel Prizes in physics. His son won the Nobel Prize in 1937 for proving the wavelike properties of electrons.
This article is licensed under the Creative Commons Attribution-ShareAlike 3.0 Unported License, which means that you can copy and modify it as long as the entire work (including additions) remains under this license.
- Loading...

-
 11:08
11:08Discovery of the Electron: Cathode Ray Tube Experiment
Discovery of the Electron: Cathode Ray Tube ExperimentDiscovery of the Electron: Cathode Ray Tube Experiment
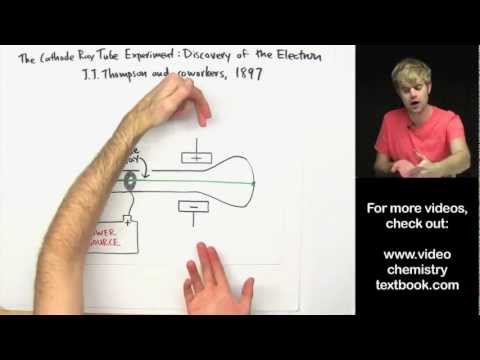
To see all my Chemistry videos, check out http://socratic.org/chemistry J.J. Thompson discovered the electron, the first of the subatomic particles, using the cathode ray tube experiment. He found that many different metals release cathode rays, and that cathode rays were made of electrons, very small negatively charged particles. This disproved John Dalton's theory of the atom, and Thompson came up with the plum pudding model of the atom. -
 38:29
38:292015-03-30 Eric Dollard on J.J. Thomson
2015-03-30 Eric Dollard on J.J. Thomson2015-03-30 Eric Dollard on J.J. Thomson
http://ericpdollard.com - http://energyscienceconference.com - J.J. Thomson is as important as Tesla but not many people understand his work. Here is a short interview on J.J. Thomson that gives some details about his work. Support Eric's Indiegogo campaign here: http://igg.me/at/ericdollard/x/2343214 -
 3:13
3:13Cathode Rays Lead to Thomson's Model of the Atom
Cathode Rays Lead to Thomson's Model of the AtomCathode Rays Lead to Thomson's Model of the Atom
In the mid 1800's scientists successfully passed an electric current through a vacuum in a glass tube. They saw a glow from the tube that seemed to emanate from the negatively charged plate called the cathode. Since scientists didn't know what the glow was they called it a cathode ray. There was debate over whether the cathode ray was a wave phenomenon like light or a stream of negatively charged particles. JJ Thomson effectively resolved the debate in 1897 by performing a clever experiment that determined the charge to mass ratio of the particles making up the cathode ray. He also showed that this same particle was in all different cathode materials so it must be a constituent common to all atoms. This changed our understanding of the atom from the previous billiard ball model to Thomson's plum pudding model of the atom. -
 2:01
2:01J. J. Thomson
J. J. ThomsonJ. J. Thomson
Thomson's contributions to cathode rays and the atomic model -
 2:49
2:49J.J. Thomson
J.J. ThomsonJ.J. Thomson
esto me lo dejaron de qimica aver si les sirve -
 1:23
1:23J. J. Thomson
J. J. Thomson -
 6:35
6:35The Plum Pudding Atomic Model, J.J. Thomson and the Electron - Chemistry
The Plum Pudding Atomic Model, J.J. Thomson and the Electron - ChemistryThe Plum Pudding Atomic Model, J.J. Thomson and the Electron - Chemistry
Mr. Causey discusses the Plum Pudding model of the atom, J.J. Thomson and the discovery of the ELECTRON. http://www.yourCHEMcoach.com Share this Video: http://www.youtube.com/watch?v=dehxVQAUqBs Learn more and understand better with Mr. Causey's tutorials. Subscribe for more chemistry videos: http://bit.ly/1jeutVl Related Videos: Solid Sphere: http://www.youtube.com/watch?v=JqCGUWue4eE Nuclear Model http://www.youtube.com/watch?v=bIVI-rMLm54 Contact Me: mrcausey@mrcausey.com Follow Me: http://www.twitter.com/#!/mrcausey http://pinterest.com/mistercausey/ http://www.facebook.com/profile.php?id=814523544 https://plus.google.com/u/0/111105504415887392612 Mr. Causey discusses the Plum Pudding model of the atom, J.J. Thomson and the discovery of the ELECTRON. Many believed that a negatively charged particle existed but no one was able to prove it. George Stoney even suggested a name for the particle, "electron". But, it wasn't till 1898 that the electron was shown to exist by J.J. Thomson performing experiments with the cathode ray tube. Thus, creating the need for a new atomic model. Thomson explained the new model as a mass of positive material with negatively charged electrons embedded in it like a plum pudding. He was awarded the Nobel Prize in 1906 for his work but it would soon be challenged by physicists such as Philip Lenard. -
 2:54
2:54The Discovery of the Electron (2 of 15)
The Discovery of the Electron (2 of 15)The Discovery of the Electron (2 of 15)
Episode 2 of In Search of Giants: Dr Brian Cox takes us on a journey through the history of particle physics. In this episode we learn how J.J. Thomson discovered the fist subatomic particle - the electron. This film is part of a series originally broadcast on Teachers' TV (http://www.teachers.tv/video/23645). The series was made with the support of The Science and Technology Facilities Council (www.scitech.ac.uk). www.lhc.ac.uk - Official UK LHC website for public and schools. www.particledetectives.net - School resources on the LHC, how science works and particle physics. Films produced and directed by Alom Shaha (www.labreporter.com). Join the conversation: Twitter: https://twitter.com/STFC_Matters Facebook: https://facebook.com/SciTechFacCouncil LinkedIn: https://www.linkedin.com/company/stfc -
 5:16
5:16Charge to mass ratio of an electron
Charge to mass ratio of an electronCharge to mass ratio of an electron
J J Thomson's experiment to determine the charge to mass ratio of an electron -
 2:49
2:49Cathode Ray Tube
Cathode Ray TubeCathode Ray Tube
Demo 10 HChem "As the cathode rays carry a charge of negative electricity, are deflected by an electrostatic force as if they were negatively electrified, and are acted on by a magnetic force in just the way in which this force would act on a negatively electrified body moving along the path of these rays, I can see no escape from the conclusion that they are charges of negative electricity carried by particles of matter." —J. J. Thomson (Philosophical Magazine, 44, 293 (1897)) -
 1:48
1:48Cathode Ray Tube
Cathode Ray TubeCathode Ray Tube
This is the official Video of Cathode Ray Tube by sir JJ Thomson.. A Cathode ray tube is the forerunner of the television tube. It is a glass tube from which most of the air has been evacuated. When the two metal plates are connected to a high-voltage source, the negatively charged plate, called the cathode, emits an invisible ray. The cathode ray is drawn to the positively charged plate, called the anode, where it passes through a hole and continues traveling to the other end of the tube. When the ray strikes the specially coated surface, the cathode ray produces a strong fluorescence, or bright light. When an electric field is applied across the cathode ray tube, the cathode ray is attracted by the plate bearing positive charges. Therefore, a cathode ray must consist of negatively charged particles. A moving charged body behaves like a tiny magnet, and it can interact with am external magnetic field. The electrons are deflected by the magnetic field. As expected, when the direction of the external magnet field is reversed, the beam of electrons is deflected in the opposite direction. In 1897, JJ Thomson, and English physicist, determined the charge-to-mass ratio of an electron. He adjusted the electric field so that the electrostatic deflection (0E) was the same as the magnetic deflection (0B), and was able to calculate the charge-to-mass ratio of an electron using the following equation: Where E is the applied electrical field, 0 is the angle of deflection, B is the applied magnetic field, and / is the distance traveled by the cathode rays. Thomson determined the charge-to-mass ratio of an electron to be -1.76 x 10 eight power coulombs per gram. hope i helped... ^^ Please Subscribe!.. and hit Like! -
 10:44
10:44J. J. Thomson's CRT Experiment - The Discovery of the Electron
J. J. Thomson's CRT Experiment - The Discovery of the Electron -
 1:50
1:50Thomson
ThomsonThomson
JJ Thomson's experiment. -
 1:10
1:10JJ Thomson featuring Eminem.dv
JJ Thomson featuring Eminem.dv
- Aage Bohr
- Aaron Klug
- Abdus Salam
- Adam Riess
- Adams Prize
- Alan Lloyd Hodgkin
- Albert Einstein
- Albert Fert
- Alexander Prokhorov
- Alfred Kastler
- Alma mater
- Amedeo Avogadro
- Andre Geim
- Andrew Huxley
- Antony Hewish
- Archibald Geikie
- Archibald Hill
- Arno Allan Penzias
- Arthur Compton
- Arthur Evans
- Arthur Schuster
- Ben Roy Mottelson
- Bertram Brockhouse
- Brian Schmidt
- Burton Richter
- C. F. Powell
- C. V. Raman
- Cambridge
- Canal rays
- Carl David Anderson
- Carl Friedrich Gauss
- Carl Wieman
- Carlo Rubbia
- Cathode ray
- Cathode ray tube
- Cathode rays
- Charles Hard Townes
- Charles K. Kao
- Charles T. R. Wilson
- Cheetham Hill
- Chen Ning Yang
- Clifford Shull
- Clinton Davisson
- Copley Medal
- Crookes tube
- Daniel C. Tsui
- David Gross
- Delta ray
- Dennis Gabor
- Dmitri Mendeleev
- Donald A. Glaser
- Douglas Hartree
- Douglas Osheroff
- Eduard Suess
- Edward John Routh
- Edward Mills Purcell
- Edwin Hubble
- Electron
- Electrons
- Emilio G. Segrè
- England
- Enrico Fermi
- Epsilon radiation
- Eric Allin Cornell
- Ernest Lawrence
- Ernest Rutherford
- Ernest Walton
- Ernst Ruska
- Erwin Schrödinger
- Eugene Wigner
- Felix Bloch
- Felix Klein
- Francis Galton
- Frank Wilczek
- Franklin Medal
- Frederick Reines
- Frederick Soddy
- Frits Zernike
- G. H. Hardy
- Gabriel Lippmann
- Galileo Galilei
- George Darwin
- George de Hevesy
- George E. Smith
- George Ellery Hale
- George Paget Thomson
- George Porter
- George Smoot
- George William Hill
- Georges Charpak
- Gerard 't Hooft
- Gerd Binnig
- Ground (electricity)
- Guglielmo Marconi
- Gustaf Dalén
- Gustav Ludwig Hertz
- H. Stanley Allen
- Hannes Alfvén
- Hans Bethe
- Hans Georg Dehmelt
- Heinrich Hertz
- Heinrich Kayser
- Heinrich Rohrer
- Hendrik Lorentz
- Henri Becquerel
- Henry Hallett Dale
- Henry Montagu Butler
- Henry Way Kendall
- Herbert Kroemer
- Hideki Yukawa
- Horace Lamb
- Horst Ludwig Störmer
- Howard Florey
- Hugh David Politzer
- Hughes Medal
- Hugo Tetrode
- Hydrogen
- Igor Tamm
- Ilya Frank
- Isaac Newton
- Isidor Isaac Rabi
- Isotope
- Isotopes
- Ivan Pavlov
- Ivar Giaever
- J. Hans D. Jensen
- J. J. Thomson
- J.H. Poynting
- Jack Kilby
- Jack Steinberger
- James Chadwick
- James Clerk Maxwell
- James Cronin
- James Dewar
- James Franck
- James Rainwater
- Jean Baptiste Perrin
- Joan Paget Thomson
- Johann Jakob Balmer
- Johannes Rydberg
- Johannes Stark
- John Bardeen
- John C. Mather
- John Cockcroft
- John Dalton
- John L. Hall
- John Napier
- John Scott Haldane
- John Zeleny
- Joseph Barcroft
- Joseph Larmor
- Joseph Stefan
- Josiah Willard Gibbs
- Julian Schwinger
- Kai Siegbahn
- Karl Ferdinand Braun
- Kenneth G. Wilson
- Klaus von Klitzing
- Knighthood
- Konstantin Novoselov
- Lancashire
- Leo Esaki
- Leon Cooper
- Leon M. Lederman
- Lev Landau
- Loránd Eötvös
- Louis de Broglie
- Louis Néel
- Ludwig Boltzmann
- Luis Walter Alvarez
- Luminiferous aether
- Manchester
- Manne Siegbahn
- Maria Goeppert-Mayer
- Marie Curie
- Martin Lewis Perl
- Martin Ryle
- Masatoshi Koshiba
- Mass spectrometer
- Mass spectrometry
- Mass-to-charge ratio
- Max Born
- Max Planck
- Max von Laue
- Melvin Schwartz
- Michael Atiyah
- Michael Faraday
- Murray Gell-Mann
- Nevill Francis Mott
- Nicolaas Bloembergen
- Niels Bohr
- Nikolay Basov
- Nobel laureate
- Nobel Prize
- Norman Lockyer
- Oswald Avery
- Otto Sackur
- Otto Stern
- Owen Chamberlain
- Owens College
- Paul Dirac
- Paul Langevin
- Paul Nurse
- Pavel Cherenkov
- Peter Debye
- Peter Grünberg
- Philipp Lenard
- Physical constant
- Physicist
- Physics
- Pierre Curie
- Pieter Zeeman
- Plum pudding model
- Polykarp Kusch
- Potassium
- Pyotr Kapitsa
- Radioactive
- Radioactivity
- Ray Lankester
- Raymond Davis, Jr.
- Riccardo Giacconi
- Richard E. Taylor
- Richard Feynman
- Robert B. Laughlin
- Robert Hofstadter
- Romanes Lecture
- Roy J. Glauber
- Royal Medal
- Royal Society
- Rudolf Mössbauer
- Russell Alan Hulse
- Samuel C. C. Ting
- Saul Perlmutter
- Scotland
- Second Wrangler
- Sharp Stewart
- Sheldon Lee Glashow
- Simon & Schuster
- Simon van der Meer
- Sin-Itiro Tomonaga
- Smith's prize
- Steven Chu
- Steven Weinberg
- T. H. Laby
- Theobald Smith
- Theodor W. Hänsch
- Thomas Hunt Morgan
- Thomson (unit)
- Thomson Problem
- Thomson problem
- Thomson scattering
- Toshihide Maskawa
- Tsung-Dao Lee
- United Kingdom
- University of Oxford
- Val Logsdon Fitch
- Victor Francis Hess
- Vitaly Ginzburg
- Walther Bothe
- Werner Heisenberg
- Westminster Abbey
- Wikipedia Link rot
- Wikipedia Persondata
- Wikisource
- Wilhelm Röntgen
- Wilhelm Wien
- Willard Boyle
- William Bayliss
- William Crookes
- William Henry Bragg
- William Huggins
- William Prout
- William Shockley
- Willis Lamb
- Wolfgang Ketterle
- Wolfgang Paul
- Wolfgang Pauli
- Yoichiro Nambu
- Zhores Alferov
- Élie Metchnikoff
- Aage Bohr
- Aaron Klug
- Abdus Salam
- Adam Riess
- Alan Lloyd Hodgkin
- Albert Einstein
- Albert Fert
- Alexander Prokhorov
- Alfred Kastler
- Amedeo Avogadro
- Andre Geim
- Andrew Huxley
- Antony Hewish
- Archibald Geikie
- Archibald Hill
- Arno Allan Penzias
- Arthur Compton
- Arthur Evans
- Arthur Schuster
- Ben Roy Mottelson
- Bertram Brockhouse
- Brian Schmidt
- Burton Richter
- Carl David Anderson
- Carl Friedrich Gauss
- Carl Wieman
- Carlo Rubbia
- Charles Hard Townes
- Chen Ning Yang
- Clifford Shull
- Clinton Davisson
- David Gross
- Dennis Gabor
- Dmitri Mendeleev
- Douglas Hartree
- Douglas Osheroff
- Eduard Suess
- Edward Mills Purcell
- Edwin Hubble
- Enrico Fermi
- Eric Allin Cornell
- Ernest Lawrence
- Ernest Rutherford
- Ernest Walton
- Ernst Ruska
- Erwin Schrödinger
- Eugene Wigner
- Felix Bloch
- Felix Klein
- Francis Galton
- Frank Wilczek
- Frederick Reines
- Frederick Soddy
- Frits Zernike
- Gabriel Lippmann
- Galileo Galilei
- George Darwin
- George de Hevesy
- George Ellery Hale
- George Paget Thomson
- George Porter
- George Smoot
- George William Hill
- Georges Charpak
- Gerd Binnig
- Guglielmo Marconi
- Gustaf Dalén
- Gustav Ludwig Hertz
- Hannes Alfvén
- Hans Bethe
- Hans Georg Dehmelt
- Heinrich Hertz
- Heinrich Kayser
- Heinrich Rohrer
- Hendrik Lorentz
- Henri Becquerel
- Henry Hallett Dale
- Henry Way Kendall
- Herbert Kroemer
- Hideki Yukawa
- Horace Lamb
- Horst Ludwig Störmer
- Howard Florey
- Hugh David Politzer
- Hugo Tetrode
- Igor Tamm
- Ilya Frank
- Isaac Newton
- Isidor Isaac Rabi
- Ivan Pavlov
- Ivar Giaever
- Jack Kilby
- Jack Steinberger
- James Chadwick
- James Clerk Maxwell
- James Cronin
- James Dewar
- James Franck
- James Rainwater
- Jean Baptiste Perrin
- Johann Jakob Balmer
- Johannes Rydberg
- Johannes Stark
- John Bardeen
- John Cockcroft
- John Dalton
- John Napier
- John Scott Haldane
- John Zeleny
- Joseph Barcroft
- Joseph Larmor
- Joseph Stefan
- Josiah Willard Gibbs
- Julian Schwinger
- Kai Siegbahn
- Karl Ferdinand Braun
- Klaus von Klitzing
- Konstantin Novoselov
- Leo Esaki
- Leon Cooper
- Lev Landau
- Loránd Eötvös
- Louis de Broglie
- Louis Néel
- Ludwig Boltzmann
- Luis Walter Alvarez
- Manne Siegbahn
- Marie Curie
- Martin Lewis Perl
- Martin Ryle
- Masatoshi Koshiba
- Max Born
- Max Planck
- Max von Laue
- Melvin Schwartz
- Michael Atiyah
- Michael Faraday
- Nevill Francis Mott
- Nicolaas Bloembergen
- Niels Bohr
- Nikolay Basov
- Norman Lockyer
- Oswald Avery
- Otto Sackur
- Otto Stern
- Owen Chamberlain
- Paul Dirac
- Paul Langevin
- Paul Nurse
- Pavel Cherenkov
- Peter Debye
- Peter Grünberg
- Philipp Lenard
- Pierre Curie
- Pieter Zeeman
- Polykarp Kusch
- Pyotr Kapitsa
- Ray Lankester
- Riccardo Giacconi
- Richard Feynman
- Robert Hofstadter
- Rudolf Mössbauer
- Russell Alan Hulse
- Saul Perlmutter
- Sheldon Lee Glashow
- Simon van der Meer
- Steven Chu
- Steven Weinberg
- Theobald Smith
- Thomas Hunt Morgan
- Toshihide Maskawa
- Val Logsdon Fitch
- Victor Francis Hess
- Vitaly Ginzburg
- Walther Bothe
- Werner Heisenberg
- Wikipedia Persondata
- Wilhelm Röntgen
- Wilhelm Wien
- Willard Boyle
- William Bayliss
- William Crookes
- William Henry Bragg
- William Huggins
- William Prout
- William Shockley
- Willis Lamb
- Wolfgang Ketterle
- Wolfgang Paul
- Wolfgang Pauli
- Yoichiro Nambu
- Zhores Alferov
- Élie Metchnikoff
-

Discovery of the Electron: Cathode Ray Tube Experiment
To see all my Chemistry videos, check out http://socratic.org/chemistry J.J. Thompson discovered the electron, the first of the subatomic particles, using the cathode ray tube experiment. He found that many different metals release cathode rays, and that cathode rays were made of electrons, very small negatively charged particles. This disproved John Dalton's theory of the atom, and Thompson came up with the plum pudding model of the atom. -

2015-03-30 Eric Dollard on J.J. Thomson
http://ericpdollard.com - http://energyscienceconference.com - J.J. Thomson is as important as Tesla but not many people understand his work. Here is a short interview on J.J. Thomson that gives some details about his work. Support Eric's Indiegogo campaign here: http://igg.me/at/ericdollard/x/2343214 -

Cathode Rays Lead to Thomson's Model of the Atom
In the mid 1800's scientists successfully passed an electric current through a vacuum in a glass tube. They saw a glow from the tube that seemed to emanate from the negatively charged plate called the cathode. Since scientists didn't know what the glow was they called it a cathode ray. There was debate over whether the cathode ray was a wave phenomenon like light or a stream of negatively charged particles. JJ Thomson effectively resolved the debate in 1897 by performing a clever experiment that determined the charge to mass ratio of the particles making up the cathode ray. He also showed that this same particle was in all different cathode materials so it must be a constituent common to all atoms. This changed our understanding of the atom from the previous billiard ball model to Thomson'... -

J. J. Thomson
Thomson's contributions to cathode rays and the atomic model -

J.J. Thomson
esto me lo dejaron de qimica aver si les sirve -

-

The Plum Pudding Atomic Model, J.J. Thomson and the Electron - Chemistry
Mr. Causey discusses the Plum Pudding model of the atom, J.J. Thomson and the discovery of the ELECTRON. http://www.yourCHEMcoach.com Share this Video: http://www.youtube.com/watch?v=dehxVQAUqBs Learn more and understand better with Mr. Causey's tutorials. Subscribe for more chemistry videos: http://bit.ly/1jeutVl Related Videos: Solid Sphere: http://www.youtube.com/watch?v=JqCGUWue4eE Nuclear Model http://www.youtube.com/watch?v=bIVI-rMLm54 Contact Me: mrcausey@mrcausey.com Follow Me: http://www.twitter.com/#!/mrcausey http://pinterest.com/mistercausey/ http://www.facebook.com/profile.php?id=814523544 https://plus.google.com/u/0/111105504415887392612 Mr. Causey discusses the Plum Pudding model of the atom, J.J. Thomson and the discovery of the ELECTRON. Many believed that a negati... -

The Discovery of the Electron (2 of 15)
Episode 2 of In Search of Giants: Dr Brian Cox takes us on a journey through the history of particle physics. In this episode we learn how J.J. Thomson discovered the fist subatomic particle - the electron. This film is part of a series originally broadcast on Teachers' TV (http://www.teachers.tv/video/23645). The series was made with the support of The Science and Technology Facilities Council (www.scitech.ac.uk). www.lhc.ac.uk - Official UK LHC website for public and schools. www.particledetectives.net - School resources on the LHC, how science works and particle physics. Films produced and directed by Alom Shaha (www.labreporter.com). Join the conversation: Twitter: https://twitter.com/STFC_Matters Facebook: https://facebook.com/SciTechFacCouncil LinkedIn: https://www.linkedin.c... -

Charge to mass ratio of an electron
J J Thomson's experiment to determine the charge to mass ratio of an electron -

Cathode Ray Tube
Demo 10 HChem "As the cathode rays carry a charge of negative electricity, are deflected by an electrostatic force as if they were negatively electrified, and are acted on by a magnetic force in just the way in which this force would act on a negatively electrified body moving along the path of these rays, I can see no escape from the conclusion that they are charges of negative electricity carried by particles of matter." —J. J. Thomson (Philosophical Magazine, 44, 293 (1897)) -

Cathode Ray Tube
This is the official Video of Cathode Ray Tube by sir JJ Thomson.. A Cathode ray tube is the forerunner of the television tube. It is a glass tube from which most of the air has been evacuated. When the two metal plates are connected to a high-voltage source, the negatively charged plate, called the cathode, emits an invisible ray. The cathode ray is drawn to the positively charged plate, called the anode, where it passes through a hole and continues traveling to the other end of the tube. When the ray strikes the specially coated surface, the cathode ray produces a strong fluorescence, or bright light. When an electric field is applied across the cathode ray tube, the cathode ray is attracted by the plate bearing positive charges. Therefore, a cathode ray must consist of negatively charg... -

-

Thomson
JJ Thomson's experiment. -

-

Thomson's Plum Pudding Model of the Atom
JJ Thomson proposed the first model of the atom with subatomic structure. He had performed a series of experiments and was credited with the discovery of the first sub-atomic particle, the electron. He therefore proposed a new model of the atom called the plum pudding model. In this model, the plums represent negatively charged electrons which can be plucked out of the atom, leaving behind some positively charged pudding. In this film, cherry tart is used as a delicious substitute for plum pudding. -

Thomson's CRT Experiment: The Discovery of the Electron
Basic breakdown of the cathode ray tube experiment performed by J. J. Thomson in the late 1800s. This experiment proved the existence of the electron--a small, negatively charged particle about 2000 times lighter than Hydrogen, the lightest known atom. -

Discovery of cathode rays and Anode rays and J.J Thomson atomic Model
Cathode rays (also called an electron beam or e-beam) are streams of electrons observed in vacuum tubes. If an evacuated glass tube is equipped with two electrodes and a voltage is applied, the glass opposite of the negative electrode is observed to glow, due to electrons emitted from and travelling perpendicular to the cathode (the electrode connected to the negative terminal of the voltage supply). They were first observed in 1869 by German physicist Johann Hittorf, and were named in 1876 by Eugen Goldstein Kathodenstrahlen, or cathode rays.[1][2] Electrons were first discovered as the constituents of cathode rays. In 1897 British physicist J. J. Thomson showed the rays were composed of a previously unknown negatively charged particle, which was later named the electron. Cathode ray tub... -

La découverte de l'électron
Conçu vers la fin du XIXe siècle, le tube cathodique permit à Joseph John Thomson de découvrir l’électron, cette particule qui compose l’atome avec le neutron et le proton. Retour en vidéo sur cette découverte qui bouleversa le monde de la connaissance scientifique. -

JJ THOMSON'S THEORY OF ATOM
Group 5 9-Lavoisier members: Gabriel Luis Rayco (editor) John Edmar Archivido Josiah Josh Benavidez Arthur David Del Rosario Adriel Jireh Lumague Vincent Philip Villar-- Created using PowToon -- Free sign up at http://www.powtoon.com/youtube/ -- Create animated videos and animated presentations for free. PowToon is a free tool that allows you to develop cool animated clips and animated presentations for your website, office meeting, sales pitch, nonprofit fundraiser, product launch, video resume, or anything else you could use an animated explainer video. PowToon's animation templates help you create animated presentations and animated explainer videos from scratch. Anyone can produce awesome animations quickly with PowToon, without the cost or hassle other professional animation servi... -

Chemistry & Physics: History of the Atom (Dalton, Thomson, Rutherford, and Bohr Models)
The model of the atom has undergone steady changes to reflect experimental results, starting with John Dalton's model (1803), to JJ Thomson's model (1897), to Ernest Ruthford's model (1909), to Niels Bohr's model (1913). Our current model, the "quantum mechanical model," will be discussed in a separate video. Our Periodic Table app is FREE in the Google Play store! http://goo.gl/yg9mAF Don't miss our other chemistry videos: https://www.youtube.com/watch?v=aQw9GqDEgeE&list;=PLi01XoE8jYoi5fLBY64f6ZUuktgTFb2H3 Please Subscribe so you'll hear about our newest videos! http://www.youtube.com/subscription_center?add_user=SocraticaStudios Written and Produced by Kimberly Hatch Harrison Creative Commons picture credits: Bohr Model http://en.wikipedia.org/wiki/File:Bohr_model_3.jpg Author: ... -

Collider: JJ Thomson's Cathode-ray tube
Content Developer Rupert Cole introduces one of the Collider exhibition's star objects: JJ Thomson's cathode-ray tube. Cambridge physicist JJ Thomson used this cathode-ray tube to investigate inside the atom. In 1897 he identified the first subatomic particle found in nature -- later known as the electron -- and sparked a new era in physics and industry. Discover more and journey inside the largest scientific experiment ever constructed in our new exhibition, Collider. Open 13 Nov 2013 - 5 May 2014 http://www.sciencemuseum.org.uk/collider -

Thomson's Model of an Atom - Class 9 Tutorial - Amrita University
In 1897, J.J. Thomson discovered the electron, the first subatomic particle. In 1904, Thomson proposed atomic model where electrons are embedded within spherically distributed, positive charge (so-called "plum pudding" model).Both the positive charge and the mass of the atom would be more or less uniformly distributed over its size. -

Trabalho de Química (Joseph John Thomson)
Joseph John Thomson
Discovery of the Electron: Cathode Ray Tube Experiment
- Order: Reorder
- Duration: 11:08
- Updated: 28 Nov 2012
- views: 146168
- published: 28 Nov 2012
- views: 146168
2015-03-30 Eric Dollard on J.J. Thomson
- Order: Reorder
- Duration: 38:29
- Updated: 14 May 2015
- views: 8034
- published: 14 May 2015
- views: 8034
Cathode Rays Lead to Thomson's Model of the Atom
- Order: Reorder
- Duration: 3:13
- Updated: 02 Feb 2011
- views: 137049
- published: 02 Feb 2011
- views: 137049
J. J. Thomson
- Order: Reorder
- Duration: 2:01
- Updated: 14 Sep 2009
- views: 136357
J.J. Thomson
- Order: Reorder
- Duration: 2:49
- Updated: 21 Sep 2010
- views: 9126
- published: 21 Sep 2010
- views: 9126
The Plum Pudding Atomic Model, J.J. Thomson and the Electron - Chemistry
- Order: Reorder
- Duration: 6:35
- Updated: 26 Nov 2010
- views: 40489
- published: 26 Nov 2010
- views: 40489
The Discovery of the Electron (2 of 15)
- Order: Reorder
- Duration: 2:54
- Updated: 01 Mar 2008
- views: 322569
- published: 01 Mar 2008
- views: 322569
Charge to mass ratio of an electron
- Order: Reorder
- Duration: 5:16
- Updated: 29 Sep 2013
- views: 26862
- published: 29 Sep 2013
- views: 26862
Cathode Ray Tube
- Order: Reorder
- Duration: 2:49
- Updated: 01 Oct 2008
- views: 226127
- published: 01 Oct 2008
- views: 226127
Cathode Ray Tube
- Order: Reorder
- Duration: 1:48
- Updated: 07 Apr 2012
- views: 169212
- published: 07 Apr 2012
- views: 169212
J. J. Thomson's CRT Experiment - The Discovery of the Electron
- Order: Reorder
- Duration: 10:44
- Updated: 02 Nov 2012
- views: 11953
Thomson
- Order: Reorder
- Duration: 1:50
- Updated: 21 Jan 2009
- views: 28828
JJ Thomson featuring Eminem.dv
- Order: Reorder
- Duration: 1:10
- Updated: 30 Apr 2010
- views: 1437
Thomson's Plum Pudding Model of the Atom
- Order: Reorder
- Duration: 2:17
- Updated: 28 Jan 2011
- views: 72643
- published: 28 Jan 2011
- views: 72643
Thomson's CRT Experiment: The Discovery of the Electron
- Order: Reorder
- Duration: 7:24
- Updated: 17 May 2011
- views: 28059
- published: 17 May 2011
- views: 28059
Discovery of cathode rays and Anode rays and J.J Thomson atomic Model
- Order: Reorder
- Duration: 38:15
- Updated: 15 Dec 2014
- views: 18433
- published: 15 Dec 2014
- views: 18433
La découverte de l'électron
- Order: Reorder
- Duration: 8:02
- Updated: 15 Sep 2014
- views: 10823
- published: 15 Sep 2014
- views: 10823
JJ THOMSON'S THEORY OF ATOM
- Order: Reorder
- Duration: 3:42
- Updated: 23 Aug 2015
- views: 90
- published: 23 Aug 2015
- views: 90
Chemistry & Physics: History of the Atom (Dalton, Thomson, Rutherford, and Bohr Models)
- Order: Reorder
- Duration: 6:32
- Updated: 28 Mar 2014
- views: 98085
- published: 28 Mar 2014
- views: 98085
Collider: JJ Thomson's Cathode-ray tube
- Order: Reorder
- Duration: 1:43
- Updated: 21 Nov 2013
- views: 2603
- published: 21 Nov 2013
- views: 2603
Thomson's Model of an Atom - Class 9 Tutorial - Amrita University
- Order: Reorder
- Duration: 4:05
- Updated: 08 Oct 2014
- views: 5144
- published: 08 Oct 2014
- views: 5144
Trabalho de Química (Joseph John Thomson)
- Order: Reorder
- Duration: 4:22
- Updated: 25 Apr 2013
- views: 894
-

Thomson, Millikan, Rutherford, and Bohr
How did J. J. Thomson, Robert Millikan, and Ernest Rutherford all help Niels Bohr came to his conclusion of the new atomic model? -

Thomson's Cathode Ray Tube Explained by @GettinJunkDone
The cathode ray tube was an experiment used by JJ Thomson to discover the electron in 1897. This was the first conclusive evidence for subatomic particles. Another good video: https://www.youtube.com/watch?v=Rb6MguN0Uj4 Be Sure to Checkout the Gettin Junk Done Stores! GJD Shirts & Gear: http://shop.spreadshirt.com/gettinjunkdone/ GJD Stickers & Notebooks: http://www.redbubble.com/people/gettinjunkdone/shop Thanks for watching! Please comment, like, subscribe and share! You can also fine me on Twitter - @GettinJunkDone Originally, Gettin Junk Done was started with the intention of making a few simple How-To videos mostly dealing with my truck and motorcycle. Once I saw that a few of my uploads were doing well, I decided to continue with additional videos as projects came up. To ... -

Nobel Prize #1 - Plum Pudding and J. J. Thomson
Using magnets, gases and metals, J.J. Thompson first found that atoms aren’t completely indivisible, but do have smaller parts. While the entire atom was natural, with ingenious experimentation, he was able to find that there was a part of an atom that was more negative; he found the electron! Here my father explains how this first Nobel prize winning scientist in our Nobel prize series discovered more about our universe. -

J. J. Thomson and the "oil-drop experiment"
Experimentor 4u - British physicist J. J. Thomson discovered the electron in 1897. Read how his work contributed to the famous "Oil Drop Experiment" at http://www.experimentor4u.com/j-j-thomson-millikans-oil-drop-experiment/. -

Modelo átomico de J.J. Thomson
-- Created using PowToon -- Free sign up at http://www.powtoon.com/youtube/ -- Create animated videos and animated presentations for free. PowToon is a free tool that allows you to develop cool animated clips and animated presentations for your website, office meeting, sales pitch, nonprofit fundraiser, product launch, video resume, or anything else you could use an animated explainer video. PowToon's animation templates help you create animated presentations and animated explainer videos from scratch. Anyone can produce awesome animations quickly with PowToon, without the cost or hassle other professional animation services require. -

J.J. Thomson
Animated Video created using Animaker - http://www.animaker.com Assignment 2.2: Atomic Model Animation Outcomes -

J. J. Thomson
J. J. Thomson =======Image-Copyright-Info======= Image is in public domain Author-Info: Not Mentioned Image Source: https://en.wikipedia.org/wiki/File:J.J_Thomson.jpg =======Image-Copyright-Info======== ☆Video is targeted to blind users Attribution: Article text available under CC-BY-SA image source in video -

J.J Thomson
-- Created using PowToon -- Free sign up at http://www.powtoon.com/youtube/ -- Create animated videos and animated presentations for free. PowToon is a free tool that allows you to develop cool animated clips and animated presentations for your website, office meeting, sales pitch, nonprofit fundraiser, product launch, video resume, or anything else you could use an animated explainer video. PowToon's animation templates help you create animated presentations and animated explainer videos from scratch. Anyone can produce awesome animations quickly with PowToon, without the cost or hassle other professional animation services require. -

Acto Carnaval JJ Thomson 2016
-

Joseph John Thomson
Biografía de Joseph John Thomson -

Joseph John Thomson
Useful for CBSE, ICSE, NCERT & International Students Grade : 8 Subject : Physics Lesson : Our Scientists Topic: Joseph John Thomson Sir J. J. Thomson was an English physicist who helped revolutionize the knowledge of atomic structure by his discovery of the electron (1897). He received the Nobel Prize for Physics in 1906 and was knighted in 1908. Visit www.oztern.com to find personalized test preparation solutions for Pre Medical - AIPMT, AIIMS, JIPMER, State, Pre Engineering - IIT JEE, JEE MAIN, BITSAT, State and Foundations - Class 6 to 10. -

علماء مشاهير- عالم الفيزياء جوزيف جون طومسون - jj.thomson
إنتاج قناة الخرج التعليمية إدارة التعليم بمحافظة الخرج -

JJ Thomson
This video is about JJ Thomson -

Live Q and A with JJ Thompson and Bethan Leadley
He’s a TV presenter who rose from the ranks of X-Factor finalist to hosting his own show on Sky, she’s a singer song writer, host of a 4Music show and a YouTube sensation with over 270k subscribers. But how did 19-year-old Bethan Leadley and 23-year-old JJ Thompson go from unknowns to success stories in just a few short years? Both are passionate about their careers and their industries and are keen to inspire young people to back themselves to achieve their dreams and take every opportunity that comes their way. Joined by a panel of young people spanning diverse careers and JJ Thompson and Bethan Leadley who will discuss the key concerns and hopes facing the youth of today and explore whether it’s harder to be a young person today than in previous generations. New research from the Ar... -

J.J. Thomson Presentation - Jenny, Vicki, and Amy
-

J. J. Thomson a British Physicist : Remembering our great scientist
Subscribe to see more. Sir Joseph John "J. J." Thomson (Lived 1856 – 1940.) J. J. Thomson took science to new heights with his 1897 discovery of the electron – the first subatomic particle. J.J. Thomson (Joseph John Thomson) was born in Cheetham Hill (a suburb of Manchester) on December 18, 1856. He is not still alive today. He went to Owens College in Manchester, in 1870. In 1876, he entered Trinity College in Cambridge as a minor scholar. Thomson studied mathematics and physics. He remained a member of Trinity College for the rest of his life and became a Lecturer in 1883 and a Master in 1918. In 1890, he married Rose Elisabeth and they had one son, (now Sir George Paget Thomson) and one daughter. J.J. Thomson died on August 30, 1940. Joseph returned to America in 1904 and deliver... -

Chem2
"History of the Atomic Theory Timeline." Timetoast. N.p., n.d. Web. 03 Dec. 2015. "Aristotle." The-History-of-the-Atom -. N.p., n.d. Web. 03 Dec. 2015. "Joseph John Thomson | Chemical Heritage Foundation." Joseph John Thomson | Chemical Heritage Foundation. N.p., n.d. Web. 03 Dec. 2015. "Atomic Theory Timeline." Atomic Theory Timeline. N.p., n.d. Web. 03 Dec. 2015. ~Democritus; 442BC ~Antoine Lavoisier; 1785 ~Joseph Louis Proust; 1799 ~John Dalton; 1803 ~Michael Faraday; 1832 ~J.J Thomson; 1897 ~Marie & Pierre Curie; 1898 ~Max Planck; 1900 ~Albert Einstein; 1905 ~Robert Millikan; 1909 ~Ernest Rutherford; 1911 ~Niels Bohr; 1922 ~Louis DeBroglie; 1924 ~James Chadwick; 1932 -

Chem project 2
"History of the Atomic Theory Timeline." Timetoast. N.p., n.d. Web. 03 Dec. 2015. "Aristotle." The-History-of-the-Atom -. N.p., n.d. Web. 03 Dec. 2015. "Joseph John Thomson | Chemical Heritage Foundation." Joseph John Thomson | Chemical Heritage Foundation. N.p., n.d. Web. 03 Dec. 2015. "Atomic Theory Timeline." Atomic Theory Timeline. N.p., n.d. Web. 03 Dec. 2015. ~Democritus; 442BC ~Antoine Lavoisier; 1785 ~Joseph Louis Proust; 1799 ~John Dalton; 1803 ~Michael Faraday; 1832 ~J.J Thomson; 1897 ~Marie & Pierre Curie; 1898 ~Max Planck; 1900 ~Albert Einstein; 1905 ~Robert Millikan; 1909 ~Ernest Rutherford; 1911 ~Niels Bohr; 1922 ~Louis DeBroglie; 1924 ~James Chadwick; 1932 -

El Experimento de Joseph John Thomson.
Unida 1: Teoría Atómica. Clase 3: El Experimento de Joseph John Thomson. Recordemos que Dalton, describió el átomo como una partícula muy pequeña he indivisible, pero investigaciones posteriores demostraron que los átomos poseen cierta estructura interna. En la década de 1890, se estudiaba la emisión y transmisión de la energía a través del espacio en forma de ondas, utilizando para ello tubos de rayos catódicos. Suscribirte: Sígueme en Twitter: @jlcastro78 E - mail: jlcastros78@gmail.com Por favor, mantengan una actitud buena y de compañerismo en la caja de comentarios. Escriban mensajes que tengan que ver principalmente con el vídeo. Eviten el spam. Recuerden que hay moderadores revisando. -

-

Joseph John Thomson Experiment (Catode Ray)
by Martin S. Silberberg Silberberg, M.S. 2012. Chemistry. The Molecular Nature of Matter and Change, 6th Edition. Mc Graw Hill, New York. -

Atom Presentation - JJ Thomson
-

Cathode Rays
Useful for CBSE, ICSE, NCERT & International Students Grade 11 Subject: Chemistry Lesson: Structure of Atom Topic: Cathode Rays Video Cathode rays (also called an electron beam or e-beam) are streams of electrons observed in vacuum tubes. Electrons were first discovered as the constituents of cathode rays. In 1897 British physicist J. J. Thomson showed the rays were composed of a previously unknown negatively charged particle, which was later named the electron. Cathode ray tubes (CRTs) use a focused beam of electrons deflected by electric or magnetic fields to create the image in a classic television set. Visit www.oztern.com to find personalized test preparation solutions for Pre Medical - AIPMT, AIIMS, JIPMER, State, Pre Engineering - IIT JEE, JEE MAIN, BITSAT, State and Foundations ...
Thomson, Millikan, Rutherford, and Bohr
- Order: Reorder
- Duration: 6:01
- Updated: 11 Mar 2016
- views: 0
- published: 11 Mar 2016
- views: 0
Thomson's Cathode Ray Tube Explained by @GettinJunkDone
- Order: Reorder
- Duration: 6:11
- Updated: 07 Mar 2016
- views: 3
- published: 07 Mar 2016
- views: 3
Nobel Prize #1 - Plum Pudding and J. J. Thomson
- Order: Reorder
- Duration: 4:50
- Updated: 28 Feb 2016
- views: 26
- published: 28 Feb 2016
- views: 26
J. J. Thomson and the "oil-drop experiment"
- Order: Reorder
- Duration: 0:45
- Updated: 14 Feb 2016
- views: 2
- published: 14 Feb 2016
- views: 2
Modelo átomico de J.J. Thomson
- Order: Reorder
- Duration: 3:57
- Updated: 10 Feb 2016
- views: 1
- published: 10 Feb 2016
- views: 1
J.J. Thomson
- Order: Reorder
- Duration: 1:35
- Updated: 09 Feb 2016
- views: 8
- published: 09 Feb 2016
- views: 8
J. J. Thomson
- Order: Reorder
- Duration: 18:54
- Updated: 06 Jan 2016
- views: 1
- published: 06 Jan 2016
- views: 1
J.J Thomson
- Order: Reorder
- Duration: 2:55
- Updated: 06 Nov 2015
- views: 4
- published: 06 Nov 2015
- views: 4
Acto Carnaval JJ Thomson 2016
- Order: Reorder
- Duration: 7:48
- Updated: 07 Feb 2016
- views: 121
- published: 07 Feb 2016
- views: 121
Joseph John Thomson
- Order: Reorder
- Duration: 2:24
- Updated: 07 Feb 2016
- views: 19
Joseph John Thomson
- Order: Reorder
- Duration: 0:48
- Updated: 14 Dec 2015
- views: 8
- published: 14 Dec 2015
- views: 8
علماء مشاهير- عالم الفيزياء جوزيف جون طومسون - jj.thomson
- Order: Reorder
- Duration: 1:28
- Updated: 24 Jan 2016
- views: 56
- published: 24 Jan 2016
- views: 56
JJ Thomson
- Order: Reorder
- Duration: 1:43
- Updated: 04 Nov 2015
- views: 6
Live Q and A with JJ Thompson and Bethan Leadley
- Order: Reorder
- Duration: 25:29
- Updated: 22 Jan 2016
- views: 284
- published: 22 Jan 2016
- views: 284
J.J. Thomson Presentation - Jenny, Vicki, and Amy
- Order: Reorder
- Duration: 3:59
- Updated: 19 Jan 2016
- views: 20
- published: 19 Jan 2016
- views: 20
J. J. Thomson a British Physicist : Remembering our great scientist
- Order: Reorder
- Duration: 1:42
- Updated: 17 Dec 2015
- views: 52
- published: 17 Dec 2015
- views: 52
Chem2
- Order: Reorder
- Duration: 1:10
- Updated: 07 Dec 2015
- views: 2
- published: 07 Dec 2015
- views: 2
Chem project 2
- Order: Reorder
- Duration: 1:10
- Updated: 04 Dec 2015
- views: 4
- published: 04 Dec 2015
- views: 4
El Experimento de Joseph John Thomson.
- Order: Reorder
- Duration: 4:01
- Updated: 23 Nov 2015
- views: 124
- published: 23 Nov 2015
- views: 124
Jj Thomson chemistry Evan Boeckman
- Order: Reorder
- Duration: 2:14
- Updated: 09 Nov 2015
- views: 7
Joseph John Thomson Experiment (Catode Ray)
- Order: Reorder
- Duration: 1:10
- Updated: 28 Oct 2015
- views: 14
- published: 28 Oct 2015
- views: 14
Atom Presentation - JJ Thomson
- Order: Reorder
- Duration: 4:31
- Updated: 27 Oct 2015
- views: 15
- published: 27 Oct 2015
- views: 15
Cathode Rays
- Order: Reorder
- Duration: 12:54
- Updated: 23 Oct 2015
- views: 12
- published: 23 Oct 2015
- views: 12
-

J.J. THOMPSON Rayos Catódicos Descubrimiento del ELECTRÓN Experimento con monitor de PC
http://www.youtube.com/user/anajesusa?sub_confirmation=1 http://espaciodecesar.com/?s=thomson Experimento de Joseph John Thomson. Rayos catódicos y descubrimiento del electrón. Una sencilla forma de adaptar un viejo monitor de ordenador para verificar las propiedades de los rayos catódicos. Un paso a paso para comprobar que estas partículas viajan en linea recta, que responden a los campos eléctricos y magnéticos y que son partículas materiales, tres de las cuatro características que descubrió el científico británico cuando descubrió el electrón. https://www.facebook.com/espaciodecesar http://espaciodecesar.com/ https://twitter.com/Espacio_cesar https://www.pinterest.com/cesarguazzaroni/ -

Podcast Physics 27-7-15 J J Thomson
A podcast from an HSC physics class on J J Thomson and his famous experiments. This is a live recording of a class intended as a revision tool for the class. Please accept that there are interruptions and the normal unplanned moments in the lesson. -

Charge and Mass of an Electron - A Level Physics
Continuing the A Level Physics revision series, this video covers, cathode rays, J J Thomson's experiment, Thermionic emission, an electron volt, measuring the charge to mass ratio for an electron and Millikan's oil drop experiment to measure the charge on an electron. -

VCE UNIT 1: History of the atom
** A few little errors in names: Thomson, not Thompson, Ernest not Ernst ** The first video of UNIT 1 VCE chemistry. A quick look at the history of chemistry and the development of atomic theory. If you want to join the class and follow along, please go to edmodo.com and join the group with the code: 9s2py4. Below is also a list of the different scientists involved and the time that I start explaining that particular scientist. Aristotle and Democritus : 8:45 John Dalton: 13:24 JJ Thomson: 15:50 Ernest Rutherford: 20:05 Niels Bohr: 23:30 Erwin Schrodinger: 27:10 James Chadwick: 29:10 Summary: 34:00 -

Química - Modelos Atômicos - EVOLUÇÃO DO MODELO ATÔMICO
Entre em contato: exatasexatas@hotmail.com Acesse o site do Exatas Exatas: http://exatasexatas.orgfree.com Vamos ver hoje a evolução dos modelos atômicos, da Grécia Antiga até o modelo de Bohr. Demócrito e Leucipo, John Dalton, J.J.Thomson, James Chadwick, Ernest Rutherford e Niels Bohr colaboraram gradativamente a melhorar o modelo de átomo, baseados em experimentos inteligentes e muito bem elaborados. Veja a experiência feita por Thomson, que comprovou a existência dos elétrons, de carga elétrica negativa (se vocês não se importarem com a voz de Google Tradutor, está bem explicado lá): http://www.youtube.com/watch?v=_Pwrvn2Zl5U ATENÇÃO: Não sou o detentor dos direitos (nem tenho a intenção de violá-los) de nenhuma música ou material de terceiros que porventura venham a ser utilizados... -

Quantum Theory Made Easy [2]
PART 1: https://www.youtube.com/watch?v=e5_V78SWGF0 Today we’ll be exploring the evolution of the atom, starting from J.J. Thomson’s Plum Pudding model, on to Rutherford’s Planetary model, and then on to the Bohr model, which is one of the central focuses of this lecture. After going over the strengths and weaknesses of each, we’ll see how de Broglie’s crazy ideas about the duality of matter manifest themselves in the Bohr model, which we’ll use to (partially) derive the Rydberg equation. Finally, after calculating the Balmer series in order to show off how successful Bohr was, I’ll ruin the moment by explaining why Bohr’s model wasn’t complete after all. Some very important questions will be raised, and the answers… well, the answers will blow your mind in the next episode. This video is... -

2015 09 06 Eric Dollard Live Call - The Power of the Aether
http://powerofaether.com - Eric Dollard on a live call answering caller's questions and addressing quite a bit of J.J. Thomson's work in regards to aether physics and other aspects of his recent presentation The Power of the Aether as Related to Music and Electricity. He also gives an update on the advanced seismic warning system. -

-

Atomic Structure: GCSE revision
GCSE level Atomic & Nuclear covering: Rutherford scattering, Rutherford, plum pudding, Dalton, Marsden, Democritus, J J Thomson, electron, alpha particle, gold foil, nuclear model, proton, neutron, charge, nucleons, ion, chemical symbol, nucleon number, proton number, mass number, atomic number -

3. Double Minima, Earnshaw's Theorem and Plum-Puddings
Freshman Organic Chemistry (CHEM 125) Continuing the discussion of Lewis structures and chemical forces from the previous lecture, Professor McBride introduces the double-well potential of the ozone molecule and its structural equilibrium. The inability for inverse-square force laws to account for stable arrangements of charged particles is prescribed by Earnshaw's Theorem, which may be visualized by means of lines of force. J.J. Thomson circumvented Earnshaw's prohibition on structure by postulating a "plum-pudding" atom. When Rutherford showed that the nucleus was a point, Thomson had to conclude that Coulomb's law was invalid at small distances. 00:00 - Chapter 1. Distinguishing Equilibrium and Resonance 06:37 - Chapter 2. The Structure and Surface Potential of Ozone 20:57 - Chapter 3... -

Astronomy Cast Ep. 377: Thomson finds Electron
Sponsored by Swinburne Astronomy Online at http://astronomy.swin.edu.au/sao/ Join +Fraser Cain and +Pamela Gay for a live episode of Astronomy Cast. We'll record our 30-minute show, and then stick around to answer your questions about space and astronomy. Astronomy Cast Ep. 377: Thomson finds Electron At the end of the 19th century, physicists were finally beginning to understand the nature of matter itself, including the discovery of electrons - tiny particles of negative charge that surround the nucleus. Here's how J.J. Thompson separated the electrons from their atoms and uncovered their nature. -

11. Orbital Correction and Plum-Pudding Molecules
Freshman Organic Chemistry (CHEM 125) The lecture opens with tricks ("Z-effective" and "Self Consistent Field") that allow one to correct approximately for the error in using orbitals that is due to electron repulsion. This error is hidden by naming it "correlation energy." Professor McBride introduces molecules by modifying J.J. Thomson's Plum-Pudding model of the atom to rationalize the form of molecular orbitals. There is a close analogy in form between the molecular orbitals of CH4 and NH3 and the atomic orbitals of neon, which has the same number of protons and neutrons. The underlying form due to kinetic energy is distorted by pulling protons out of the Ne nucleus to play the role of H atoms. 00:00 - Chapter 1. Introduction 01:53 - Chapter 2. Correcting for Electron Repulsion when ... -

Kafa Ayarı #20 - Tüplü TV ve CRT Monitörden Bugüne Ekranlar
Karşısına kilitlenip kaldığımız bu ekranları kim icat etti? Kafa Ayarı #20'de katot ışık tüplerinden bugüne ekranları ve gözlerimiz halini konuşuyoruz... Abone olmak için ► http://goo.gl/5QcJiR Bir zamanlar karşısında oturup ailecek TRT izlediğimiz tüplü televizyonlar vardı. Önce bu televizyonlara Atari'lerimizi bağlamaya başladık, ardından masamızın üzerindeki bilgisayarımızın ekranına kilitlendik. Bugün neredeyse hepimizin elinde yine bir ekran var, akıllı cep telefonları. Peki kimin fikriydi bu ilk ekran? CRT yani Cathode Ray Tube: Katot Işın Tüpü ile başladığımız sohbetimize buyrun. Soğuyan havanın da etkisiyle sıcak birer kuşburnu eşliğinde CRT'den LCD'ye, Monochrome'dan Plazma'ya kadar farklı ekran türleri, teknolojileri ve bize yaşattıklarını konuşuyoruz. Sıcak içeceğiniz eşliğind... -

Jim Al-Khalili - Quantum Life: How Physics Can Revolutionise Biology
In this Friday Evening Discourse at the Royal Institution, Professor Jim Al-Khalili explores how the mysteries of quantum theory might be observable at the biological level. Although many examples can be found in the scientific literature dating back half a century, there is still no widespread acceptance that quantum mechanics -- that baffling yet powerful theory of the subatomic world -- might play an important role in biological processes. Biology is, at its most basic, chemistry, and chemistry is built on the rules of quantum mechanics in the way atoms and molecules behave and fit together. As Jim explains, biologists have until recently been dismissive of counter-intuitive aspects of the theory and feel it to be unnecessary, preferring their traditional ball-and-stick models of th... -

Podcast CP Chem I Ch.3.2a
JJ Thomson, Ernest Rutherford, subatomic particles, gold foil experiment, cathode ray tube -

Atomic Theory and Elements
This video covers the following: 1. Definition of an element, atom, and compound. 2. How elements are named and element symbols. 3. Early atomic models: Democritus 4. Dalton's postulates 5. Law of constant composition. 6. J.J. Thomson's experiment. 7. Plum Pudding model of the atom 8. Millikan's oil drop experiment. 9. Rutherford's (scattering, gold foil) experiment. 10. Properties of the subatomic particles. 11. Atomic number 12. Mass number 13. Isotope -

General Chemistry Lecture: History of Atomic Theory Part 2
Discovery of electron. J. J. Thomson's CRT experiments. Millikan's oil drop experiment. Rutherford's alpha scattering experiment. CORRECTION: at 10:00, the magnetic field vector should be pointing in the opposite direction. -

Delirio su "Chi Vuol Essere Milionario"
Camtasia ha registrato male il video, mi scuso per gli attacchi epilettici che potreste aver subito! NB: Ho confuso Rutherford con J.J. Thomson, spero mi perdoniate.. VUOI LA PARTNER?? eccola http://awe.sm/iGByZ Link pagina FB https://www.facebook.com/pages/Valerion19-taddy93/362470880548834 PSN taddy93 REGISTRO CON ELGATO GAME CAPTURE HD http://www.elgato.com/gaming/game-capture-hd -

Ri Discourse: Jon Butterworth - High energy physics at the LHC
In this Friday Evening Discourse at the Royal Institution, Professor Jon Butterworth, member of the High Energy Physics group on the Atlas experiment, provides an overview of his work at the Large Hadron Collider (LHC). Friday Evening Discourses The tradition of Friday evening discourses at the Royal Institution was started by Michael Faraday in 1825. Since that time most major scientific figures have spoken in the famous Lecture Theatre at the heart of the Ri building at 21 Albemarle Street. Notable talks include Faraday announcing the existence of the technology of photography in 1839 and J.J. Thomson announcing the existence of the fundamental particle later called the electron in 1897. This Ri Discourse took place on Friday 3 February 2012. The Ri is on Twitter: http://twitter.com/... -

Electronics: Waveguide Plumbing 1979 US Air Force Training Film
more at http://scitech.quickfound.net "Covers the 90 E and H bend in rectangular waveguides by explaining the effect on the field within the guide. twists, flexible waveguide, and the effects of improper bends are discussed. The choke joint and rotating joint are explained both physically and electrically. The actions of the feedhorn and dummy load are explained by mockup and cellomatic visuals. Actual bends, twists, choke joints, flexible waveguide, feedhorn and dummy load are shown." Electronics playlist: https://www.youtube.com/playlist?list=PLAA9B0175C3E15B47 USAF Training Film TV 906 Air Training Command. Public domain film from the US National Archives, slightly cropped to remove uneven edges, with the aspect ratio corrected, and mild video noise reduction applied. The soundtrack...
J.J. THOMPSON Rayos Catódicos Descubrimiento del ELECTRÓN Experimento con monitor de PC
- Order: Reorder
- Duration: 21:07
- Updated: 05 Jun 2014
- views: 7896
- published: 05 Jun 2014
- views: 7896
Podcast Physics 27-7-15 J J Thomson
- Order: Reorder
- Duration: 32:46
- Updated: 27 Jul 2015
- views: 3
- published: 27 Jul 2015
- views: 3
Charge and Mass of an Electron - A Level Physics
- Order: Reorder
- Duration: 23:16
- Updated: 21 Mar 2012
- views: 22927
- published: 21 Mar 2012
- views: 22927
VCE UNIT 1: History of the atom
- Order: Reorder
- Duration: 38:05
- Updated: 06 Dec 2012
- views: 6312
- published: 06 Dec 2012
- views: 6312
Química - Modelos Atômicos - EVOLUÇÃO DO MODELO ATÔMICO
- Order: Reorder
- Duration: 25:19
- Updated: 03 Feb 2013
- views: 68730
- published: 03 Feb 2013
- views: 68730
Quantum Theory Made Easy [2]
- Order: Reorder
- Duration: 35:25
- Updated: 01 Dec 2015
- views: 50428
- published: 01 Dec 2015
- views: 50428
2015 09 06 Eric Dollard Live Call - The Power of the Aether
- Order: Reorder
- Duration: 81:30
- Updated: 08 Oct 2015
- views: 2797
- published: 08 Oct 2015
- views: 2797
MODELO ATOMICO DE THOMSON
- Order: Reorder
- Duration: 21:01
- Updated: 10 Jan 2013
- views: 19466
Atomic Structure: GCSE revision
- Order: Reorder
- Duration: 22:03
- Updated: 29 Jan 2014
- views: 6032
- published: 29 Jan 2014
- views: 6032
3. Double Minima, Earnshaw's Theorem and Plum-Puddings
- Order: Reorder
- Duration: 45:03
- Updated: 18 Sep 2009
- views: 16529
- published: 18 Sep 2009
- views: 16529
Astronomy Cast Ep. 377: Thomson finds Electron
- Order: Reorder
- Duration: 53:42
- Updated: 18 May 2015
- views: 1774
- published: 18 May 2015
- views: 1774
11. Orbital Correction and Plum-Pudding Molecules
- Order: Reorder
- Duration: 49:27
- Updated: 18 Sep 2009
- views: 5512
- published: 18 Sep 2009
- views: 5512
Kafa Ayarı #20 - Tüplü TV ve CRT Monitörden Bugüne Ekranlar
- Order: Reorder
- Duration: 22:16
- Updated: 09 Oct 2014
- views: 24855
- published: 09 Oct 2014
- views: 24855
Jim Al-Khalili - Quantum Life: How Physics Can Revolutionise Biology
- Order: Reorder
- Duration: 59:58
- Updated: 30 Jan 2013
- views: 239129
- published: 30 Jan 2013
- views: 239129
Podcast CP Chem I Ch.3.2a
- Order: Reorder
- Duration: 41:52
- Updated: 23 Sep 2014
- views: 14
- published: 23 Sep 2014
- views: 14
Atomic Theory and Elements
- Order: Reorder
- Duration: 27:39
- Updated: 16 Sep 2014
- views: 159
- published: 16 Sep 2014
- views: 159
General Chemistry Lecture: History of Atomic Theory Part 2
- Order: Reorder
- Duration: 37:37
- Updated: 09 Apr 2013
- views: 293
- published: 09 Apr 2013
- views: 293
Delirio su "Chi Vuol Essere Milionario"
- Order: Reorder
- Duration: 30:08
- Updated: 17 Oct 2013
- views: 7011
- published: 17 Oct 2013
- views: 7011
Ri Discourse: Jon Butterworth - High energy physics at the LHC
- Order: Reorder
- Duration: 61:41
- Updated: 07 Feb 2012
- views: 4403
- published: 07 Feb 2012
- views: 4403
Electronics: Waveguide Plumbing 1979 US Air Force Training Film
- Order: Reorder
- Duration: 26:00
- Updated: 23 Sep 2014
- views: 2902
- published: 23 Sep 2014
- views: 2902
- Playlist
- Chat
- Playlist
- Chat

Discovery of the Electron: Cathode Ray Tube Experiment
- Report rights infringement
- published: 28 Nov 2012
- views: 146168

2015-03-30 Eric Dollard on J.J. Thomson
- Report rights infringement
- published: 14 May 2015
- views: 8034

Cathode Rays Lead to Thomson's Model of the Atom
- Report rights infringement
- published: 02 Feb 2011
- views: 137049

J. J. Thomson
- Report rights infringement
- published: 14 Sep 2009
- views: 136357

J.J. Thomson
- Report rights infringement
- published: 21 Sep 2010
- views: 9126


The Plum Pudding Atomic Model, J.J. Thomson and the Electron - Chemistry
- Report rights infringement
- published: 26 Nov 2010
- views: 40489

The Discovery of the Electron (2 of 15)
- Report rights infringement
- published: 01 Mar 2008
- views: 322569

Charge to mass ratio of an electron
- Report rights infringement
- published: 29 Sep 2013
- views: 26862

Cathode Ray Tube
- Report rights infringement
- published: 01 Oct 2008
- views: 226127

Cathode Ray Tube
- Report rights infringement
- published: 07 Apr 2012
- views: 169212

J. J. Thomson's CRT Experiment - The Discovery of the Electron
- Report rights infringement
- published: 02 Nov 2012
- views: 11953

JJ Thomson featuring Eminem.dv
- Report rights infringement
- published: 30 Apr 2010
- views: 1437
- Playlist
- Chat

Thomson, Millikan, Rutherford, and Bohr
- Report rights infringement
- published: 11 Mar 2016
- views: 0

Thomson's Cathode Ray Tube Explained by @GettinJunkDone
- Report rights infringement
- published: 07 Mar 2016
- views: 3

Nobel Prize #1 - Plum Pudding and J. J. Thomson
- Report rights infringement
- published: 28 Feb 2016
- views: 26

J. J. Thomson and the "oil-drop experiment"
- Report rights infringement
- published: 14 Feb 2016
- views: 2

Modelo átomico de J.J. Thomson
- Report rights infringement
- published: 10 Feb 2016
- views: 1

J.J. Thomson
- Report rights infringement
- published: 09 Feb 2016
- views: 8

J. J. Thomson
- Report rights infringement
- published: 06 Jan 2016
- views: 1

J.J Thomson
- Report rights infringement
- published: 06 Nov 2015
- views: 4

Acto Carnaval JJ Thomson 2016
- Report rights infringement
- published: 07 Feb 2016
- views: 121

Joseph John Thomson
- Report rights infringement
- published: 07 Feb 2016
- views: 19

Joseph John Thomson
- Report rights infringement
- published: 14 Dec 2015
- views: 8

علماء مشاهير- عالم الفيزياء جوزيف جون طومسون - jj.thomson
- Report rights infringement
- published: 24 Jan 2016
- views: 56

JJ Thomson
- Report rights infringement
- published: 04 Nov 2015
- views: 6

Live Q and A with JJ Thompson and Bethan Leadley
- Report rights infringement
- published: 22 Jan 2016
- views: 284
- Playlist
- Chat

J.J. THOMPSON Rayos Catódicos Descubrimiento del ELECTRÓN Experimento con monitor de PC
- Report rights infringement
- published: 05 Jun 2014
- views: 7896

Podcast Physics 27-7-15 J J Thomson
- Report rights infringement
- published: 27 Jul 2015
- views: 3

Charge and Mass of an Electron - A Level Physics
- Report rights infringement
- published: 21 Mar 2012
- views: 22927

VCE UNIT 1: History of the atom
- Report rights infringement
- published: 06 Dec 2012
- views: 6312

Química - Modelos Atômicos - EVOLUÇÃO DO MODELO ATÔMICO
- Report rights infringement
- published: 03 Feb 2013
- views: 68730

Quantum Theory Made Easy [2]
- Report rights infringement
- published: 01 Dec 2015
- views: 50428

2015 09 06 Eric Dollard Live Call - The Power of the Aether
- Report rights infringement
- published: 08 Oct 2015
- views: 2797

MODELO ATOMICO DE THOMSON
- Report rights infringement
- published: 10 Jan 2013
- views: 19466

Atomic Structure: GCSE revision
- Report rights infringement
- published: 29 Jan 2014
- views: 6032

3. Double Minima, Earnshaw's Theorem and Plum-Puddings
- Report rights infringement
- published: 18 Sep 2009
- views: 16529

Astronomy Cast Ep. 377: Thomson finds Electron
- Report rights infringement
- published: 18 May 2015
- views: 1774

11. Orbital Correction and Plum-Pudding Molecules
- Report rights infringement
- published: 18 Sep 2009
- views: 5512

Kafa Ayarı #20 - Tüplü TV ve CRT Monitörden Bugüne Ekranlar
- Report rights infringement
- published: 09 Oct 2014
- views: 24855

Jim Al-Khalili - Quantum Life: How Physics Can Revolutionise Biology
- Report rights infringement
- published: 30 Jan 2013
- views: 239129
Porn and the Threat to Virility
Edit Time Magazine 31 Mar 2016Kim Kardashian, Emily Ratajkowski post topless bathroom pic
Edit New York Daily News 30 Mar 2016At Least 21 Confirmed Dead, Scores Injured, Missing In Kolkata Overpass Collapse
Edit WorldNews.com 31 Mar 2016Fukushima Nuclear Plant Operator Throws Switch For Ice Wall Around Damaged Reactors
Edit WorldNews.com 31 Mar 2016Archaeologists Find Rare Etruscan Stone From 2,500 years Ago in Florence
Edit WorldNews.com 30 Mar 2016New Thomson Reuters Tax Research Offers Guidance to Tax Practitioners Preparing for Puerto Rico’s Value-Added Tax | Thomson Reuters (Thomson Reuters Corporation)
Edit Public Technologies 31 Mar 2016Reuters Enriches Offering to Media Express Users by Adding Red Bull Media House Content | Thomson Reuters (Thomson Reuters Corporation)
Edit Public Technologies 31 Mar 2016Notice Concerning Recognition Received at DEALWATCH AWARD 2015 by Thomson Reuters(113.2KB) (Japan Hotel REIT Investment Corporation)
Edit Public Technologies 31 Mar 2016New Thomson Reuters Tax Research Offers Guidance to Tax Practitioners Preparing for Puerto Rico's Value-Added Tax
Edit Stockhouse 31 Mar 2016Child beggar to hero: videogame shows suffering of Senegal street children
Edit Yahoo Daily News 31 Mar 2016Electronic passport gates may make child trafficking easier: UK campaigners
Edit Yahoo Daily News 31 Mar 2016Thousands may die from drought in Somalia unless donors give more: U.N
Edit Yahoo Daily News 31 Mar 2016March 31, 2016 - City of Guelph responds to statement of claim (City of Guelph)
Edit Public Technologies 31 Mar 2016Indians mobilize to break free from forced, bonded labor - Harvard study
Edit Yahoo Daily News 31 Mar 2016Tui Travel reports strong UK demand despite terrorism and weaker pound
Edit The Guardian 31 Mar 2016After-hours buzz: Micron, Medivation, EPE & more
Edit Yahoo Daily News 31 Mar 2016Kayak review: Battle for the high ground a tale of two classes
Edit Sydney Morning Herald 31 Mar 2016- 1
- 2
- 3
- 4
- 5
- Next page »







