- published: 06 Aug 2012
- views: 4883
-
remove the playlistStable Isotope
- remove the playlistStable Isotope
- published: 10 Feb 2015
- views: 1701
- published: 03 Jan 2013
- views: 6166
- published: 31 Jul 2014
- views: 263
- published: 01 Feb 2016
- views: 160
- published: 21 Jan 2015
- views: 428
- published: 07 Jan 2016
- views: 41

Stable isotopes are chemical isotopes that may or may not be radioactive, but if radioactive, have half-lives too long to be measured.
Only 90 nuclides from the first 40 elements are energetically stable to any kind of decay save proton decay, in theory (see list of nuclides). An additional 164 are theoretically unstable to known types of decay, but no evidence of decay has ever been observed, for a total of 254 nuclides for which there is no evidence of radioactivity. By this definition, there are 254 known stable nuclides of the 80 elements which have one or more stable isotopes. A list of these is given at the end of this article.
Of the 80 elements with one or more stable isotopes, twenty-six have only a single stable isotope, and are thus termed monoisotopic, and the rest have more than one stable isotope. One element (tin) has ten stable isotopes, the largest number known for an element.
Different isotopes of the same element (whether stable or unstable) have nearly the same chemical characteristics and therefore behave almost identically in biology (a notable exception is the isotopes of hydrogen—see heavy water). The mass differences, due to a difference in the number of neutrons, will result in partial separation of the light isotopes from the heavy isotopes during chemical reactions and during physical processes such as diffusion and vaporization. This process is called isotope fractionation. For example, the difference in mass between the two stable isotopes of hydrogen, 1H (1 proton, no neutron, also known as protium) and 2H (1 proton, 1 neutron, also known as deuterium) is almost 100%. Therefore, a significant fractionation will occur.
This article is licensed under the Creative Commons Attribution-ShareAlike 3.0 Unported License, which means that you can copy and modify it as long as the entire work (including additions) remains under this license.
- Loading...

-
 1:35
1:35Stable Isotopes 1
Stable Isotopes 1 -
 61:56
61:56Stable Isotopes Lecture
Stable Isotopes LectureStable Isotopes Lecture
Introduction to stable isotope fractionation, why isotopes fractionate and why fractionation is temperature dependent -
 45:05
45:05Earth History, Stable Isotope Stratigraphy
Earth History, Stable Isotope StratigraphyEarth History, Stable Isotope Stratigraphy
http://www.world-earthquakes.com -
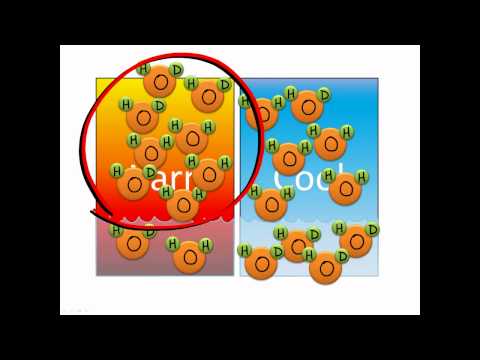 16:53
16:53Isotope fractionation
Isotope fractionationIsotope fractionation
An introduction to isotope fractionation -
 4:50
4:50"Belt of Stability": Is the isotope stable?
"Belt of Stability": Is the isotope stable?"Belt of Stability": Is the isotope stable?
How can we guess what KIND of nuclear reaction will happen? How do we know if a newly-formed isotope is stable? The Belt of stability shows where the "sweet spot" of proton/neutron ratio is. Too high = Too many neutrons = beta radiation Too low = Too many protons = positron emission More than 84 protons = alpha particle emission Check me out: http://www.chemistnate.com -
 11:57
11:57Jen McWhorter: Creation of a Stable Isotope Map of the California Coast
Jen McWhorter: Creation of a Stable Isotope Map of the California CoastJen McWhorter: Creation of a Stable Isotope Map of the California Coast
Stable isotope maps, or isoscapes, have proven invaluable in terrestrial ecology. But what about in the oceans? Jennifer McWhorter pioneers a marine isoscape for Southern California in hopes of better understanding the lives of ocean dwellers. Jennifer is an MAS graduate of the Center for Marine Biodiversity and Conservation at Scripps Institution of Oceanography. -
 0:50
0:50Stable Isotope Analysis
Stable Isotope Analysis -
 38:06
38:06Introduction to the stable isotope Lecture
Introduction to the stable isotope LectureIntroduction to the stable isotope Lecture
Video introduction to why stable isotopes are fractionated by chemical reactions. to be watched before the stable isotope lecture -
 8:58
8:589th IsoEcol Conference 2014 - on the Applications of Stable Isotope Techniques to Ecological Studies
9th IsoEcol Conference 2014 - on the Applications of Stable Isotope Techniques to Ecological Studies9th IsoEcol Conference 2014 - on the Applications of Stable Isotope Techniques to Ecological Studies
The 9th International Conference on the Applications of Stable Isotope Techniques to Ecological Studies (9the IsoEcol) has been held between 3 and 8 August 2014 at The University of Western Australia. IsoEcol is organized biannually and brings together researchers from universities, industry and government with interests in the development and application of stable isotope techniques to the ecological sciences. The field of stable isotope ecology, and the technology that underpins this research, has expanded tremendously and is now an essential part of the toolbox of each ecologist and environmental scientist. During the 9th IsoEcol more than 120 talks and posters have been presented, covering every single aspect of ecology, encompassing ecosystems from the open ocean to the suburbs of cities. http://isoecol2014.org -
 5:04
5:04Stable Isotope Geochemistry Laboratory
Stable Isotope Geochemistry LaboratoryStable Isotope Geochemistry Laboratory
This video describes one of the two Stable Isotope Facilities, available at The Open University, Milton Keynes, UK. The system, based around Thermo MAT 253 mass spectrometers, is a compound specific isotope ratio mass spectrometer system. http://www.europlanet-2020-ri.eu/europlanet-2020-ri/distributed-sample-analysis-facility-dsaf Acknowledgement: Europlanet 2020 RI has received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No 654208 Europlanet 2020 RI http://www.europlanet-2020-ri.eu/ Europlanet Outreach http://www.europlanet-eu.org/ Europlanet Social Media: Twitter: https://twitter.com/europlanetmedia Facebook: https://www.facebook.com/europlanetmedia Instagram: https://instagram.com/europlanetmedia Flickr: https://www.flickr.com/photos/europlanetmedia LinkedIn: https://www.linkedin.com/company/europlanet-2020-ri
- Alpha particle
- Aluminium-27
- Antimony-121
- Antimony-123
- Argon-36
- Argon-38
- Argon-40
- Arsenic-75
- Barium-132
- Barium-134
- Barium-135
- Barium-136
- Barium-137
- Barium-138
- Beryllium-9
- Beta decay
- Big Bang
- Bismuth-209
- Boron-10
- Boron-11
- Bromine-79
- Bromine-81
- Cadmium-106
- Cadmium-108
- Cadmium-110
- Cadmium-111
- Cadmium-112
- Cadmium-114
- Caesium-133
- Calcium-40
- Calcium-42
- Calcium-43
- Calcium-44
- Calcium-46
- Carbon
- Carbon-12
- Carbon-13
- Carbon-14
- Cerium-136
- Cerium-138
- Cerium-140
- Cerium-142
- Chemical element
- Chlorine-35
- Chlorine-37
- Chromium-50
- Chromium-52
- Chromium-53
- Chromium-54
- Cluster decay
- Cobalt-59
- Copper
- Copper-63
- Copper-65
- Cosmic ray
- Cosmic rays
- Cosmogenic nuclide
- Decay chain
- Deuterium
- Double beta decay
- Dysprosium-156
- Dysprosium-158
- Dysprosium-160
- Dysprosium-161
- Dysprosium-162
- Dysprosium-163
- Dysprosium-164
- Electron capture
- Erbium-162
- Erbium-164
- Erbium-166
- Erbium-167
- Erbium-168
- Erbium-170
- Europium-153
- Fluorine-19
- Francium
- Gadolinium-154
- Gadolinium-155
- Gadolinium-156
- Gadolinium-157
- Gadolinium-158
- Gadolinium-160
- Gallium-69
- Gallium-71
- Germanium-70
- Germanium-72
- Germanium-73
- Germanium-74
- Gold-197
- Hafnium-176
- Hafnium-177
- Hafnium-178
- Hafnium-179
- Hafnium-180
- Half-life
- Heavy water
- Helium-3
- Helium-4
- Holmium-165
- Hydrogen
- Hydrogen-1
- Hydrogen-2
- Indium-113
- Iodine-127
- Iridium-191
- Iridium-193
- Iron
- Iron-54
- Iron-56
- Iron-57
- Iron-58
- Island of stability
- Isomeric transition
- Isotope
- Isotope geochemistry
- Krypton-78
- Krypton-80
- Krypton-82
- Krypton-83
- Krypton-84
- Krypton-86
- Lanthanum-138
- Lanthanum-139
- Lead
- Lead-204
- Lead-206
- Lead-207
- Lead-208
- List of nuclides
- Lithium-6
- Lithium-7
- Lutetium-175
- Magnesium-24
- Magnesium-25
- Magnesium-26
- Manganese-55
- Mass spectrometry
- Mercury-196
- Mercury-198
- Mercury-199
- Mercury-200
- Mercury-201
- Mercury-202
- Mercury-204
- Molybdenum
- Molybdenum-92
- Molybdenum-94
- Molybdenum-95
- Molybdenum-96
- Molybdenum-97
- Molybdenum-98
- Monoisotopic element
- Mononuclidic element
- Neodymium-142
- Neodymium-143
- Neodymium-145
- Neodymium-146
- Neodymium-148
- Neon-20
- Neon-21
- Neon-22
- Neutrons
- Nickel-58
- Nickel-60
- Nickel-61
- Nickel-62
- Nickel-64
- Niobium-92
- Niobium-93
- Nitrogen
- Nitrogen-14
- Nitrogen-15
- Nuclear isomer
- Nuclear shell model
- Nucleosynthesis
- Nuclide
- Osmium-184
- Osmium-187
- Osmium-188
- Osmium-189
- Osmium-190
- Osmium-192
- Oxygen
- Oxygen-16
- Oxygen-17
- Oxygen-18
- Palladium-102
- Palladium-104
- Palladium-105
- Palladium-106
- Palladium-108
- Palladium-110
- Periodic table
- Phosphorus-31
- Platinum-192
- Platinum-194
- Platinum-195
- Platinum-196
- Platinum-198
- Plutonium-244
- Polonium
- Potassium-39
- Potassium-40
- Potassium-41
- Praseodymium-141
- Primordial nuclide
- Promethium
- Protium
- Proton decay
- Radioactive
- Radiogenic
- Radiometric dating
- Radionuclide
- Radium
- Rhenium-185
- Rhodium-103
- Rubidium-85
- Ruthenium-100
- Ruthenium-101
- Ruthenium-102
- Ruthenium-104
- Ruthenium-96
- Ruthenium-98
- Ruthenium-99
- Samarium-144
- Samarium-149
- Samarium-150
- Samarium-152
- Samarium-154
- Scandium-45
- Selenium-74
- Selenium-76
- Selenium-77
- Selenium-78
- Selenium-80
- Silicon-28
- Silicon-29
- Silicon-30
- Silver-107
- Silver-109
- Sodium-23
- Solar System
- Spontaneous fission
- Stable isotope
- Stable nuclide
- Strontium-84
- Strontium-86
- Strontium-87
- Strontium-88
- Sulfur
- Sulfur-32
- Sulfur-33
- Sulfur-34
- Sulfur-36
- Table of nuclides
- Tantalum-180m
- Tantalum-181
- Technetium
- Tellurium-120
- Tellurium-122
- Tellurium-123
- Tellurium-124
- Tellurium-125
- Tellurium-126
- Terbium-159
- Thallium-203
- Thallium-205
- Thorium
- Thulium-169
- Tin
- Tin-112
- Tin-114
- Tin-115
- Tin-116
- Tin-117
- Tin-118
- Tin-119
- Tin-120
- Tin-122
- Tin-124
- Titanium-46
- Titanium-47
- Titanium-48
- Titanium-49
- Titanium-50
- Tungsten-182
- Tungsten-183
- Tungsten-184
- Tungsten-186
- Uranium-235
- Vanadium-50
- Vanadium-51
- Xenon
- Xenon-124
- Xenon-126
- Xenon-128
- Xenon-129
- Xenon-130
- Xenon-131
- Xenon-132
- Xenon-134
- Ytterbium-168
- Ytterbium-170
- Ytterbium-171
- Ytterbium-172
- Ytterbium-173
- Ytterbium-174
- Ytterbium-176
- Yttrium-89
- Zinc
- Zinc-64
- Zinc-66
- Zinc-67
- Zinc-68
- Zinc-70
- Zirconium-90
- Zirconium-91
- Zirconium-92
- Zirconium-94
-

-

Stable Isotopes Lecture
Introduction to stable isotope fractionation, why isotopes fractionate and why fractionation is temperature dependent -

Earth History, Stable Isotope Stratigraphy
http://www.world-earthquakes.com -

Isotope fractionation
An introduction to isotope fractionation -

"Belt of Stability": Is the isotope stable?
How can we guess what KIND of nuclear reaction will happen? How do we know if a newly-formed isotope is stable? The Belt of stability shows where the "sweet spot" of proton/neutron ratio is. Too high = Too many neutrons = beta radiation Too low = Too many protons = positron emission More than 84 protons = alpha particle emission Check me out: http://www.chemistnate.com -

Jen McWhorter: Creation of a Stable Isotope Map of the California Coast
Stable isotope maps, or isoscapes, have proven invaluable in terrestrial ecology. But what about in the oceans? Jennifer McWhorter pioneers a marine isoscape for Southern California in hopes of better understanding the lives of ocean dwellers. Jennifer is an MAS graduate of the Center for Marine Biodiversity and Conservation at Scripps Institution of Oceanography. -

-

Introduction to the stable isotope Lecture
Video introduction to why stable isotopes are fractionated by chemical reactions. to be watched before the stable isotope lecture -

9th IsoEcol Conference 2014 - on the Applications of Stable Isotope Techniques to Ecological Studies
The 9th International Conference on the Applications of Stable Isotope Techniques to Ecological Studies (9the IsoEcol) has been held between 3 and 8 August 2014 at The University of Western Australia. IsoEcol is organized biannually and brings together researchers from universities, industry and government with interests in the development and application of stable isotope techniques to the ecological sciences. The field of stable isotope ecology, and the technology that underpins this research, has expanded tremendously and is now an essential part of the toolbox of each ecologist and environmental scientist. During the 9th IsoEcol more than 120 talks and posters have been presented, covering every single aspect of ecology, encompassing ecosystems from the open ocean to the suburbs of cit... -

Stable Isotope Geochemistry Laboratory
This video describes one of the two Stable Isotope Facilities, available at The Open University, Milton Keynes, UK. The system, based around Thermo MAT 253 mass spectrometers, is a compound specific isotope ratio mass spectrometer system. http://www.europlanet-2020-ri.eu/europlanet-2020-ri/distributed-sample-analysis-facility-dsaf Acknowledgement: Europlanet 2020 RI has received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No 654208 Europlanet 2020 RI http://www.europlanet-2020-ri.eu/ Europlanet Outreach http://www.europlanet-eu.org/ Europlanet Social Media: Twitter: https://twitter.com/europlanetmedia Facebook: https://www.facebook.com/europlanetmedia Instagram: https://instagram.com/europlanetmedia Flickr: https://www.flick...
Stable Isotopes 1
- Order: Reorder
- Duration: 1:35
- Updated: 06 Aug 2012
- views: 4883
Stable Isotopes Lecture
- Order: Reorder
- Duration: 61:56
- Updated: 10 Feb 2015
- views: 1701
- published: 10 Feb 2015
- views: 1701
Earth History, Stable Isotope Stratigraphy
- Order: Reorder
- Duration: 45:05
- Updated: 31 Oct 2013
- views: 1421
Isotope fractionation
- Order: Reorder
- Duration: 16:53
- Updated: 04 Aug 2014
- views: 5371
- published: 04 Aug 2014
- views: 5371
"Belt of Stability": Is the isotope stable?
- Order: Reorder
- Duration: 4:50
- Updated: 03 Jan 2013
- views: 6166
- published: 03 Jan 2013
- views: 6166
Jen McWhorter: Creation of a Stable Isotope Map of the California Coast
- Order: Reorder
- Duration: 11:57
- Updated: 31 Jul 2014
- views: 263
- published: 31 Jul 2014
- views: 263
Stable Isotope Analysis
- Order: Reorder
- Duration: 0:50
- Updated: 27 Mar 2016
- views: 81
Introduction to the stable isotope Lecture
- Order: Reorder
- Duration: 38:06
- Updated: 01 Feb 2016
- views: 160
- published: 01 Feb 2016
- views: 160
9th IsoEcol Conference 2014 - on the Applications of Stable Isotope Techniques to Ecological Studies
- Order: Reorder
- Duration: 8:58
- Updated: 21 Jan 2015
- views: 428
- published: 21 Jan 2015
- views: 428
Stable Isotope Geochemistry Laboratory
- Order: Reorder
- Duration: 5:04
- Updated: 07 Jan 2016
- views: 41
- published: 07 Jan 2016
- views: 41
- Playlist
- Chat
- Playlist
- Chat

Stable Isotopes 1
- Report rights infringement
- published: 06 Aug 2012
- views: 4883

Stable Isotopes Lecture
- Report rights infringement
- published: 10 Feb 2015
- views: 1701

Earth History, Stable Isotope Stratigraphy
- Report rights infringement
- published: 31 Oct 2013
- views: 1421

Isotope fractionation
- Report rights infringement
- published: 04 Aug 2014
- views: 5371

"Belt of Stability": Is the isotope stable?
- Report rights infringement
- published: 03 Jan 2013
- views: 6166

Jen McWhorter: Creation of a Stable Isotope Map of the California Coast
- Report rights infringement
- published: 31 Jul 2014
- views: 263


Introduction to the stable isotope Lecture
- Report rights infringement
- published: 01 Feb 2016
- views: 160

9th IsoEcol Conference 2014 - on the Applications of Stable Isotope Techniques to Ecological Studies
- Report rights infringement
- published: 21 Jan 2015
- views: 428

Stable Isotope Geochemistry Laboratory
- Report rights infringement
- published: 07 Jan 2016
- views: 41
Around 50,000 people attend Orlando shooting vigil Sunday night
Edit WPTV 20 Jun 2016China dog meat festival: Is it really so bad to eat dog?
Edit CNN 20 Jun 2016If Hillary Is Elected Should Americans Expect More "Financial" Warfare Swarming?
Edit WorldNews.com 20 Jun 2016Trump Thinks The U.S. Should ‘Start Thinking About’ Racial Profiling
Edit TPM 20 Jun 2016‘Go out; make a difference’ (University of Cape Town)
Edit Public Technologies 17 Jun 2016Turkeys on the fringe (The University of New Mexico)
Edit Public Technologies 09 Jun 2016Responding to change: Isotope analysis tackles prehistoric adaptation to agrarian lifestyle (The University of Georgia)
Edit Public Technologies 09 Jun 2016How El Niño impacts global temperatures » (The Australian National University)
Edit Public Technologies 09 Jun 2016‘Pristine’ landscapes haven’t existed for thousands of years due to human activity (University of Oxford)
Edit Public Technologies 08 Jun 2016'Pristine' landscapes haven’t existed for thousands of years (University of Oxford)
Edit Public Technologies 07 Jun 2016‘Pristine’ landscapes haven’t existed for thousands of years due to human activity (Max-Planck-Gesellschaft zur Förderung der Wissenschaften eV)
Edit Public Technologies 07 Jun 2016World Environment Day: Using Nuclear Science to Help Halt Illegal Trade in Ivory and Timber (IAEA - International Atomic Energy Agency)
Edit Public Technologies 05 Jun 2016Underwater 'lost city' found to be geological formation
Edit Deccan Chronicle 04 Jun 2016Iran, Russia eye producing stable isotopes
Edit Topix 01 Jun 2016Iran to Produce Stable Isotopes at Fordow Site
Edit Sputnik 01 Jun 2016Europe after 20 tons of Iran heavy water: AEOI chief
Edit Press TV 31 May 2016No heavy water for US without upfront payment: Iran's atomic agency
Edit Press TV 31 May 2016- 1
- 2
- 3
- 4
- 5
- Next page »







